Long-term safety and tolerability of valsartan in children aged 6 to 17 years with hypertension
- PMID: 30397789
- PMCID: PMC6349801
- DOI: 10.1007/s00467-018-4114-0
Long-term safety and tolerability of valsartan in children aged 6 to 17 years with hypertension
Abstract
Objective: The present study aimed to assess the long-term safety and tolerability of valsartan in hypertensive children aged 6-17 years, with or without chronic kidney disease (CKD).
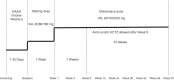
Methods: This was an 18-month, open-label, multicentre, prospective study conducted in 150 patients with history of hypertension with or without CKD. The primary endpoint was long-term safety and tolerability of valsartan and valsartan-based treatments, assessed in terms of adverse events (AEs), serious AEs, laboratory measurements, estimated glomerular filtration rate (eGFR), urinalysis and electrocardiogram.
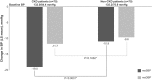
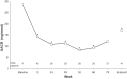
Results: Of 150 enrolled patients, 117 (78%) completed the study. At week 78, a clinically and statistically significant reduction in mean sitting systolic and diastolic blood pressures was observed in all patients (- 14.9 mmHg and - 10.6 mmHg, respectively). Within the first 3 months of treatment, mean urine albumin creatinine ratio decreased in CKD population, which was sustained. A higher percentage of CKD patients had at least one AE compared to non-CKD patients (85.3% vs. 73.3%, respectively). The majority of AEs were mild (50.7%) or moderate (18.7%) in severity. As expected, in patients with underlying CKD, increases in serum potassium, creatinine and blood urea nitrogen were more commonly reported compared to non-CKD patients. A > 25% decrease in Schwartz eGFR was observed in 28.4% of CKD patients and 13.5% of non-CKD patients.
Conclusions: Valsartan was generally well tolerated, with an AE profile consistent with angiotensin receptor blockers in the overall population and in patients with underlying CKD. Long-term efficacy was maintained and a beneficial effect on proteinuria was observed.
Keywords: Chronic kidney disease; Hypertension; Long-term safety; Paediatric; Valsartan.
Conflict of interest statement
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
RLM has participated as principal investigator in this study funded by Novartis Pharma AG. BS, ZA, EZ, HKY and HGK declare they do not have any conflict of interest. MT, RG and MAV are employees of Novartis Pharmaceuticals Corporation and have ownership in Novartis stock. LW is an employee of Novartis Pharmaceuticals Corporation.
Figures

References
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. - PubMed
-
- Hasan DM, Emeash AH, Mustafa SB, Abdelazim GE, El-din AA. Hypertension in Egypt: a systematic review. Curr Hypertens Rev. 2014;10:134–141. - PubMed
-
- Ostchega Y, Carroll M, Prineas RJ, McDowell MA, Louis T, Tilert T. Trends of elevated blood pressure among children and adolescents: data from the National Health and Nutrition Examination Survey 1988-2006. Am J Hypertens. 2009;22:59–67. - PubMed
-
- Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

