RNA interference of a trehalose-6-phosphate synthase gene reveals its roles in the biosynthesis of chitin and lipids in Heortia vitessoides (Lepidoptera: Crambidae)
- PMID: 30397994
- PMCID: PMC7379938
- DOI: 10.1111/1744-7917.12650
RNA interference of a trehalose-6-phosphate synthase gene reveals its roles in the biosynthesis of chitin and lipids in Heortia vitessoides (Lepidoptera: Crambidae)
Abstract
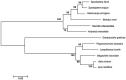
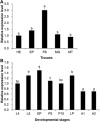
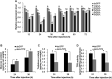
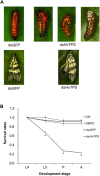
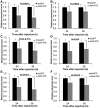
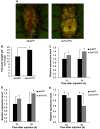
Trehalose-6-phosphate synthase (TPS), an enzyme that hydrolyzes two glucose molecules to yield trehalose, plays a pivotal role in various physiological processes. In this study, we cloned the trehalose-6-phosphate synthase gene (HvTPS) and investigated its expression patterns in various tissues and developmental stages in Heortia vitessoides Moore (Lepidoptera: Crambidae). HvTPS was highly expressed in the fat body and after pupation or before molting. We knocked down TPS in H. vitessoides by RNA interference and found that 3.0 μg of dsHvTPS resulted in optimal interference at 24 h and 36 h post-injection and caused a sharp decline in the survival rate during the 5th instar larval-pupal stage and obviously abnormal or lethal phenotypes. Additionally, compared to the controls, TPS activity and trehalose contents were significantly lower and the glucose content was significantly higher 24 h or 36 h after injection with 3.0 μg of dsHvTPS. Furthermore, the silencing of HvTPS suppressed the expression of six key genes in the chitin biosynthesis pathway and one key gene related to lipid catabolism. The expression levels of two genes associated with lipid biosynthesis were upregulated. These results strongly suggest that HvTPS is essential for the normal growth and development of H. vitessoides and provide a reference for further studies of the utility of key genes involved in chitin and lipid biosynthesis for controlling insect development.
Keywords: Heortia vitessoides Moore; RNA interference; chitin biosynthesis; lipid biosynthesis; metamorphosis; trehalose-6-phosphate synthase.
© 2018 Institute of Zoology, Chinese Academy of Sciences.
Figures
References
-
- Arakane, Y. , Muthukrishnan, S. , Kramer, K.J. , Specht, C.A. , Tomoyasu, Y. , Lorenzen, M.D . et al (2005) The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Molecular Biology, 14, 453–463. - PubMed
-
- Becker, A. , Schlöder, P. , Steele, J.E. and Wegener, G. (1996) The regulation of trehalose metabolism in insects. Experientia, 52, 433–439. - PubMed
-
- Birch, G.G. (1962) Trehalose. Advances in Carbohydrate Chemistry, 18, 201–225. - PubMed
-
- Candy, D.J. and Kilby, B.A. (1962) Studies on chitin synthesis in the desert locust. Journal of Experimental Biology, 39, 129–140.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

