Pharmacogenetics to prevent heparin-induced thrombocytopenia: what do we know?
- PMID: 30398086
- PMCID: PMC6854440
- DOI: 10.2217/pgs-2018-0147
Pharmacogenetics to prevent heparin-induced thrombocytopenia: what do we know?
Abstract
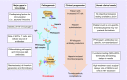
Heparin-induced thrombocytopenia (HIT) is a life-threatening, immune-mediated adverse reaction to heparin anticoagulants. The inability to predict HIT represents a considerable liability associated with heparin administration. Genetic studies of HIT are challenging due to the scarcity of true HIT cases, potential for misclassification, and many environmental risk factors. Genetic studies have not consistently identified risk alleles for HIT, the production of platelet factor 4/heparin antibodies or the thromboembolic complications of HIT. Genes implicated in HIT and platelet factor 4/heparin antibody levels include FCGR2A, TDAG8, HLA-DR and others. Compelling evidence also suggests that the FCGR2A H131R polymorphism is associated with HIT-related thrombosis. There is a need for well-powered, multiethnic studies with laboratory confirmation of HIT, detailed patient- and drug-specific data, and inclusion of both serologic and thromboembolic outcomes. Genomic biomarkers identified from such studies offer the possibility of shifting current clinical practice paradigms from early detection and treatment to prevention.
Keywords: anticoagulant; biomarker; genetics; genome-wide association study; heparin; heparin-induced thrombocytopenia; low molecular weight heparin; pharmacogenomics.
Conflict of interest statement
JH Karnes receives support from the American Heart Association (16SDG29090005) and the American College of Clinical Pharmacy Research Institute (Futures Grant). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N. Engl. J. Med. 1995;332(20):1330–1335. - PubMed
-
- Girolami B, Prandoni P, Stefani PM, et al. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: a prospective cohort study. Blood. 2003;101(8):2955–2959. - PubMed
-
- Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. a retrospective analysis of 408 patients. Thromb. Haemost. 2005;94(1):132–135. - PubMed
-
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–2715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
