The proto CpG island methylator phenotype of sessile serrated adenomas/polyps
- PMID: 30398409
- PMCID: PMC6342079
- DOI: 10.1080/15592294.2018.1543504
The proto CpG island methylator phenotype of sessile serrated adenomas/polyps
Abstract
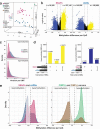
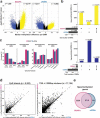
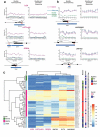
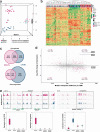
Sessile serrated adenomas/polyps (SSA/Ps) are the putative precursors of the ~20% of colon cancers with the CpG island methylator phenotype (CIMP). To investigate the epigenetic phenotype of these precancers, we prospectively collected fresh-tissue samples of 17 SSA/Ps and 15 conventional adenomas (cADNs), each with a matched sample of normal mucosa. Their DNA was subjected to bisulfite next-generation sequencing to assess methylation levels at ~2.7 million CpGs located predominantly in gene regulatory regions and spanning 80.5Mb; RNA was sequenced to define the samples' transcriptomes. Compared with normal mucosa, SSA/Ps and cADNs exhibited markedly remodeled methylomes. In cADNs, hypomethylated regions were far more numerous (18,417 vs 4288 in SSA/Ps) and rarely affected CpG islands/shores. SSA/Ps seemed to have escaped this wave of demethylation. Cytosine hypermethylation in SSA/Ps was more pervasive (hypermethylated regions: 22,147 vs 15,965 in cADNs; hypermethylated genes: 4938 vs 3443 in cADNs) and more extensive (region for region), and it occurred mainly within CpG islands and shores. Given its resemblance to the CIMP typical of SSA/Ps' putative descendant colon cancers, we refer to the SSA/P methylation phenotype as proto-CIMP. Verification studies of six hypermethylated regions in an independent series of precancers demonstrated DNA methylation markers' high potential for predicting the diagnosis of SSA/Ps and cADNs. Surprisingly, proto-CIMP in SSA/Ps was associated with upregulated gene expression; downregulation was more common in cADNs. In conclusion, the epigenetic landscape of SSA/Ps differs markedly from that of cADNs. These differences are a potentially rich source of novel tissue-based and noninvasive biomarkers.
Keywords: DNA methylation; Sessile serrated adenoma/polyp; adenomatous polyp; colon cancer.
Figures

References
-
- Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005. August;2(8):398–405. PubMed PMID: 16130936. - PubMed
-
- Ijspeert JEG, Vermeulen L, Meijer GA, et al. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015. July;12(7):401–409. . PubMed PMID: 25963511. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
