Tandem Repeats Contribute to Coding Sequence Variation in Bumblebees (Hymenoptera: Apidae)
- PMID: 30398620
- PMCID: PMC6286909
- DOI: 10.1093/gbe/evy244
Tandem Repeats Contribute to Coding Sequence Variation in Bumblebees (Hymenoptera: Apidae)
Abstract
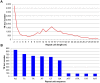
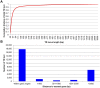
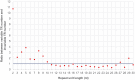
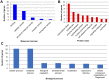
Tandem repeats (TRs) are highly dynamic regions of the genome. Mutations at these loci represent a significant source of genetic variation and can facilitate rapid adaptation. Bumblebees are important pollinating insects occupying a wide range of habitats. However, to date, molecular mechanisms underlying the potential adaptation of bumblebees to diverse habitats are largely unknown. In the present study, we investigate how TRs contribute to genetic variation in bumblebees, thus potentially facilitating adaptation. We identified 26,595 TRs from the assembled 18 chromosome sequences of the buff-tailed bumblebee (Bombus terrestris), 66.7% of which reside in genic regions. We also compared TRs found in B. terrestris with those present in the assembled genome sequence of a congener, B. impatiens. We found that a total of 1,137 TRs were variable in length between the two sequenced bumblebee species, and further analysis reveals that 101 of them are located within coding regions. These 101 TRs are responsible for coding sequence variation and correspond to protein sequence length variation between the two bumblebee species. The variability of identified TRs in coding regions between bumblebees was confirmed by PCR amplification of a subset of loci. Functional classification of bumblebee genes where coding sequences include variable-length TRs suggests that a majority of genes (87%) that could be assigned to a protein class are related to transcriptional regulation. Our results show that TRs contribute to coding sequence variation in bumblebees, and thus may facilitate the adaptation of bumblebees through diversifying proteins involved in controlling gene expression.
Figures



Similar articles
-
Genome-wide identification of accessible chromatin regions in bumblebee by ATAC-seq.Sci Data. 2020 Oct 26;7(1):367. doi: 10.1038/s41597-020-00713-w. Sci Data. 2020. PMID: 33106500 Free PMC article.
-
The genomes of two key bumblebee species with primitive eusocial organization.Genome Biol. 2015 Apr 24;16(1):76. doi: 10.1186/s13059-015-0623-3. Genome Biol. 2015. PMID: 25908251 Free PMC article.
-
Analysis of a normalised expressed sequence tag (EST) library from a key pollinator, the bumblebee Bombus terrestris.BMC Genomics. 2010 Feb 15;11:110. doi: 10.1186/1471-2164-11-110. BMC Genomics. 2010. PMID: 20156341 Free PMC article.
-
Microsporidia: An Emerging Threat to Bumblebees?Trends Parasitol. 2017 Oct;33(10):754-762. doi: 10.1016/j.pt.2017.06.001. Epub 2017 Jun 26. Trends Parasitol. 2017. PMID: 28663099 Review.
-
Genome (in)stability at tandem repeats.Semin Cell Dev Biol. 2021 May;113:97-112. doi: 10.1016/j.semcdb.2020.10.003. Epub 2020 Oct 24. Semin Cell Dev Biol. 2021. PMID: 33109442 Review.
Cited by
-
Evolution of plastid genomes of Holcoglossum (Orchidaceae) with recent radiation.BMC Evol Biol. 2019 Feb 26;19(1):63. doi: 10.1186/s12862-019-1384-5. BMC Evol Biol. 2019. PMID: 30808310 Free PMC article.
-
Advancing genomic technologies and clinical awareness accelerates discovery of disease-associated tandem repeat sequences.Genome Res. 2022 Jan;32(1):1-27. doi: 10.1101/gr.269530.120. Epub 2021 Dec 29. Genome Res. 2022. PMID: 34965938 Free PMC article. Review.
-
On the wrong DNA track: Molecular mechanisms of repeat-mediated genome instability.J Biol Chem. 2020 Mar 27;295(13):4134-4170. doi: 10.1074/jbc.REV119.007678. Epub 2020 Feb 14. J Biol Chem. 2020. PMID: 32060097 Free PMC article. Review.
-
Hidden genomic evolution in a morphospecies-The landscape of rapidly evolving genes in Tetrahymena.PLoS Biol. 2019 Jun 3;17(6):e3000294. doi: 10.1371/journal.pbio.3000294. eCollection 2019 Jun. PLoS Biol. 2019. PMID: 31158217 Free PMC article.
-
Fast sequence-based microsatellite genotyping development workflow.PeerJ. 2020 May 4;8:e9085. doi: 10.7717/peerj.9085. eCollection 2020. PeerJ. 2020. PMID: 32411534 Free PMC article.
References
-
- Barrett RD, Schluter D.. 2008. Adaptation from standing genetic variation. Trends Ecol Evol. 23(1):38–44. - PubMed
-
- Caburet S, Cocquet J, Vaiman D, Veitia RA.. 2005. Coding repeats and evolutionary “agility”. Bioessays 27(6):581–587. - PubMed
-
- Cameron SA, Hines HM, Williams PH.. 2007. A comprehensive phylogeny of the bumble bees (Bombus). Biol J Linn Soc. 91(1):161–188.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

