Reflexive Laboratory-Based Cryptococcal Antigen Screening and Preemptive Fluconazole Therapy for Cryptococcal Antigenemia in HIV-Infected Individuals With CD4 <100 Cells/µL: A Stepped-Wedge, Cluster-Randomized Trial
- PMID: 30399034
- PMCID: PMC6339522
- DOI: 10.1097/QAI.0000000000001894
Reflexive Laboratory-Based Cryptococcal Antigen Screening and Preemptive Fluconazole Therapy for Cryptococcal Antigenemia in HIV-Infected Individuals With CD4 <100 Cells/µL: A Stepped-Wedge, Cluster-Randomized Trial
Abstract
Background: HIV-infected persons with cryptococcal antigenemia (CrAg) are at high risk for meningitis or death. We evaluated the effect of CrAg screening and preemptive fluconazole therapy, adjunctive to antiretroviral therapy (ART), on 6-month survival among persons with advanced HIV/AIDS.
Methods: We enrolled HIV-infected, ART-naive participants with <100 CD4 cells/µL, in a stepped-wedge, cluster-randomized trial from July 2012 to December 2014 at 17 Ugandan clinics. Clinics participated in a prospective observational phase, followed by an interventional phase with laboratory-based, reflexive CrAg screening of residual CD4 count plasma. Asymptomatic CrAg+ participants received preemptive fluconazole therapy. We assessed 6-month survival using Cox-regression, adjusting for nadir CD4, calendar time, and stepped-wedge steps.
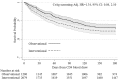
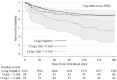
Results: We included 1280 observational and 2108 interventional participants, of whom 9.3% (195/2108) were CrAg+. CD4-, time-, and stepped-wedge-adjusted analyses demonstrated no difference in survival in the observational vs the interventional arms (hazard ratio = 1.34; 95% confidence interval: 0.86 to 2.10; P = 0.20). Fewer participants initiated ART in the interventional (73%) versus the observational phase (82%, P < 0.001). When ART initiation was modeled as a time-dependent covariate or confounder, survival did not differ. However, 6-month mortality of participants with CrAg titers <1:160 and CrAg-negative patients did not differ. Patients with CrAg titers ≥1:160 had 2.6-fold higher 6-month mortality than patients with titers <1:160.
Conclusions: We observed no overall survival benefit of the CrAg screen-and-treat intervention. However, preemptive antifungal therapy for asymptomatic cryptococcosis seemed to be effective in patients with CrAg titer <1:160. A more aggressive approach is required for persons with CrAg titer ≥1:160.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures


References
-
- Park BJ, Shetty S, Ahlquist A, et al. Long-term follow-up and survival of antiretroviral-naive patients with cryptococcal meningitis in the pre-antiretroviral therapy era, Gauteng Province, South Africa. Int J STD AIDS. 2011;22:199–203. - PubMed
-
- Park BJ, Wannemuehler KA, Marston BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

