Arrhythmia initiation in catecholaminergic polymorphic ventricular tachycardia type 1 depends on both heart rate and sympathetic stimulation
- PMID: 30399185
- PMCID: PMC6219810
- DOI: 10.1371/journal.pone.0207100
Arrhythmia initiation in catecholaminergic polymorphic ventricular tachycardia type 1 depends on both heart rate and sympathetic stimulation
Abstract
Aims: Catecholaminergic polymorphic ventricular tachycardia type 1 (CPVT1) predisposes to ventricular tachyarrhythmias (VTs) during high heart rates due to physical or psychological stress. The essential role of catecholaminergic effects on ventricular cardiomyocytes in this situation is well documented, but the importance of heart rate per se for arrhythmia initiation in CPVT1 is largely unexplored.
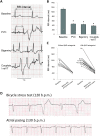
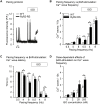
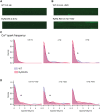
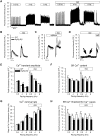
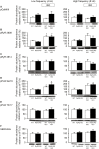
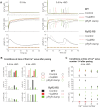
Methods and results: Sixteen CPVT1 patients performed a bicycle stress-test. Occurrence of VT triggers, i.e. premature ventricular complexes (PVC), depended on high heart rate, with individual thresholds. Atrial pacing above the individual PVC threshold in three patients did not induce PVCs. The underlying mechanism for the clinical observation was explored using cardiomyocytes from mice with the RyR2-R2474S (RyR2-RS) mutation, which exhibit exercise-induced VTs. While rapid pacing increased the number of Ca2+ waves in both RyR2-RS and wild-type (p<0.05), β-adrenoceptor (βAR) stimulation induced more Ca2+ waves in RyR2-RS (p<0.05). Notably, Ca2+ waves occurred despite decreased sarcoplasmic reticulum (SR) Ca2+ content in RyR2-RS (p<0.05), suggesting increased cytosolic RyR2 Ca2+ sensitivity. A computational model of mouse ventricular cardiomyocyte electrophysiology reproduced the cellular CPVT1 phenotype when RyR2 Ca2+ sensitivity was increased. Importantly, diastolic fluctuations in phosphorylation of RyR2 and SR Ca2+ content determined Ca2+ wave initiation. These factors were modulated towards increased propensity for arrhythmia initiation by increased pacing rates, but even more by βAR stimulation.
Conclusion: In CPVT1, VT propensity depends on individual heart rate thresholds for PVCs. Through converging data from clinical exercise stress-testing, cellular studies and computational modelling, we confirm the heart rate-independent pro-arrhythmic effects of βAR stimulation in CPVT1, but also identify an independent and synergistic contribution from effects of high heart rate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Lehnart SE, Wehrens XHT, Laitinen PiJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109(25):3208–14. 10.1161/01.CIR.0000132472.98675.EC - DOI - PubMed
-
- Khoury A, Marai I, Suleiman M, Blich M, Lorber A, Gepstein L, et al. Flecainide therapy suppresses exercise-induced ventricular arrhythmias in patients with CASQ2-associated catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10(11):1671–5. 10.1016/j.hrthm.2013.08.011 - DOI - PubMed
-
- Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106(1):69–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

