Towards a Unified Model of SMC Complex Function
- PMID: 30399354
- PMCID: PMC6850909
- DOI: 10.1016/j.cub.2018.08.034
Towards a Unified Model of SMC Complex Function
Abstract
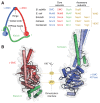
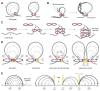
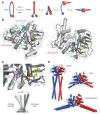
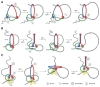
Protein complexes built of structural maintenance of chromosomes (SMC) and kleisin subunits, including cohesin, condensin and the Smc5/6 complex, are master organizers of genome architecture in all kingdoms of life. How these large ring-shaped molecular machines use the energy of ATP hydrolysis to change the topology of chromatin fibers has remained a central unresolved question of chromosome biology. A currently emerging concept suggests that the common principle that underlies the essential functions of SMC protein complexes in the control of gene expression, chromosome segregation or DNA damage repair is their ability to expand DNA into large loop structures. Here, we review the current knowledge about the biochemical and structural properties of SMC protein complexes that might enable them to extrude DNA loops and compare their action to other motor proteins and nucleic acid translocases. We evaluate the currently predominant models of active loop extrusion and propose a detailed version of a 'scrunching' model, which reconciles much of the available mechanistic data and provides an elegant explanation for how SMC protein complexes fulfill an array of seemingly diverse tasks during the organization of genomes.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

References
-
- Cobbe N, Heck MMS. The evolution of ATPase activity in SMC proteins. Proteins. 2006;63:685–96. - PubMed
-
- Gruber S. MukBEF on the march: taking over chromosome organization in bacteria? Mol Microbiol. 2011;81:855–859. - PubMed
-
- Uhlmann F. SMC complexes: from DNA to chromosomes. Nat Rev Mol Cell Biol. 2016;17:399–412. - PubMed
-
- van Ruiten MS, Rowland BD. SMC Complexes: Universal DNA Looping Machines with Distinct Regulators. Trends Genet. 2018;34:477–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

