GnRH antagonist treatment of malignant adrenocortical tumors
- PMID: 30400009
- PMCID: PMC6215908
- DOI: 10.1530/ERC-17-0399
GnRH antagonist treatment of malignant adrenocortical tumors
Abstract
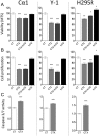
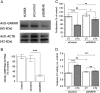
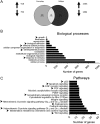
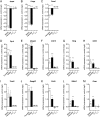
Aberrantly expressed G protein-coupled receptors in tumors are considered as potential therapeutic targets. We analyzed the expressions of receptors of gonadotropin-releasing hormone (GNRHR), luteinizing hormone/chorionic gonadotropin (LHCGR) and follicle-stimulating hormone (FSHR) in human adrenocortical carcinomas and assessed their response to GnRH antagonist therapy. We further studied the effects of the GnRH antagonist cetrorelix acetate (CTX) on cultured adrenocortical tumor (ACT) cells (mouse Cα1 and Y-1, and human H295R), and in vivo in transgenic mice (SV40 T-antigen expression under inhibin α promoter) bearing Lhcgr and Gnrhr in ACT. Both models were treated with control (CT), CTX, human chorionic gonadotropin (hCG) or CTX+hCG, and their growth and transcriptional changes were analyzed. In situ hybridization and qPCR analysis of human adrenocortical carcinomas (n = 11-13) showed expression of GNRHR in 54/73%, LHCGR in 77/100% and FSHR in 0%, respectively. CTX treatment in vitro decreased cell viability and proliferation, and increased caspase 3/7 activity in all treated cells. In vivo, CTX and CTX+hCG (but not hCG alone) decreased ACT weights and serum LH and progesterone concentrations. CTX treatment downregulated the tumor markers Lhcgr and Gata4. Upregulated genes included Grb10, Rerg, Nfatc and Gnas, all recently found to be abundantly expressed in healthy adrenal vs ACT. Our data suggest that CTX treatment may improve the therapy of human adrenocortical carcinomas by direct action on GNRHR-positive cancer cells inducing apoptosis and/or reducing gonadotropin release, directing tumor cells towards a healthy adrenal gene expression profile.
Keywords: GNRHR; GnRH antagonist; LHCGR; cetrorelix; therapy.
Figures



References
-
- Albiger N, Sartorato P, Mariniello B, Iacobone M, Finco I, Fassina A, Mantero F.2011. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? European Journal of Endocrinology of the European Federation of Endocrine Societies 164 405–412. (10.1530/EJE-10-0879) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

