Accumulation of Dinophysis Toxins in Bivalve Molluscs
- PMID: 30400229
- PMCID: PMC6266557
- DOI: 10.3390/toxins10110453
Accumulation of Dinophysis Toxins in Bivalve Molluscs
Abstract
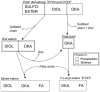
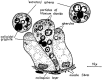
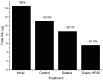
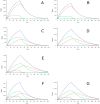
Several species of the dinoflagellate genus Dinophysis produce toxins that accumulate in bivalves when they feed on populations of these organisms. The accumulated toxins can lead to intoxication in consumers of the affected bivalves. The risk of intoxication depends on the amount and toxic power of accumulated toxins. In this review, current knowledge on the main processes involved in toxin accumulation were compiled, including the mechanisms and regulation of toxin acquisition, digestion, biotransformation, compartmentalization, and toxin depuration. Finally, accumulation kinetics, some models to describe it, and some implications were also considered.
Keywords: Dinophysis toxins; accumulation; biotransformation; compartmentalization; depuration; digestion; kinetics; okadaic acid; pectenotoxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978;44:1249–1255. doi: 10.2331/suisan.44.1249. - DOI
-
- Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of toxic shellfish in Japan and chemical properties of the toxin. In: Taylor D.L., Seliger H.W., editors. Toxic Dinoflagellate Blooms. Elsevier; New York, NY, USA: 1979. pp. 395–398.
-
- Murata M., Shimatani M., Sugitani H., Oshima Y., Yasumoto T. Isolation and structural elucidation of the causative toxin of diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982;48:549–552. doi: 10.2331/suisan.48.549. - DOI
-
- Tachibana K., Scheuer P., Tsukitani Y., Kikuchi H., Enden V., Clardy J., Gopichand Y., Schmitz F. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981;103:2469–2471. doi: 10.1021/ja00399a082. - DOI
-
- Tangen K. Shellfish poisoning and the ocurrence of potentially toxic dinoflagellates in Norwegian waters. Sarsia. 1983;68:1–7. doi: 10.1080/00364827.1983.10420550. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

