Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjögren Syndrome
- PMID: 30400384
- PMCID: PMC6274910
- DOI: 10.3390/ijms19113463
Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjögren Syndrome
Abstract
Purpose: Sjögren syndrome (SS) is a chronic inflammatory autoimmune disease of the lacrimal and salivary glands. This study compared the concentrations of epidermal fatty-acid binding protein (E-FABP) in the saliva, serum, and tears of SS patients with dry eye and dry mouth, with those of healthy adults to investigate the usefulness of E-FABP as a diagnostic marker for SS.
Design: Prospective, observational case series.
Participants: The subjects were 11 new patients with untreated Sjogren syndrome and 12 healthy control individuals.
Methods: The diagnosis of SS was in accordance with the Ministry of Health, Labour and Welfare (Japan) Diagnostic Criteria (1999). Saliva, serum, and tear specimens were collected during internal medicine, dental, and ophthalmological examinations. The ophthalmological tests included the Dry Eye-related Quality of life Score (DEQS), tear break-up time (BUT), vital staining with fluorescein (FS) and lissamine green (LG), and the Schirmer test-1. The E-FABP concentration in the tears, saliva, and serum was measured by enzyme-linked immunosorbent assay (ELISA).
Main outcome measure: The E-FABP concentrations were compared between patients and controls.
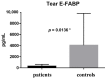
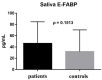
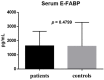
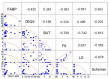
Results: There were significant differences between the patient and healthy control groups in all ophthalmological test results. There were no significant differences between the groups in the E-FABP concentrations in the saliva (p = 0.1513) or the serum (p = 0.4799), but the E-FABP concentration in the tears significantly differed between groups. The E-FABP concentration in tears tended to be significantly lower in patients with SS (mean, 323.5 ± 325.6 pg/mL) than healthy control subjects (mean, 4076 pg/mL; p = 0.0136). The E-FABP concentration in tears significantly correlated with the results of dry eye parameters.
Conclusion: The E-FABP concentration in tears appears to be related to ocular surface epithelial damage and tear stability and may be a promising novel biomarker in the diagnosis of SS.
Keywords: dry eye; epidermal fatty-acid binding protein (E-FABP), Sjögren’s syndrome; tears.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Salivary and Lacrimal Gland Alterations of the Epidermal Fatty Acid-Binding Protein (E-FABP) in Non-Obese Diabetic Mice.Int J Mol Sci. 2022 Mar 23;23(7):3491. doi: 10.3390/ijms23073491. Int J Mol Sci. 2022. PMID: 35408851 Free PMC article.
-
Differential Diagnosis of Sjögren Versus Non-Sjögren Dry Eye Through Tear Film Biomarkers.Cornea. 2020 Aug;39(8):991-997. doi: 10.1097/ICO.0000000000002299. Cornea. 2020. PMID: 32195754
-
Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients.Am J Ophthalmol. 2013 Aug;156(2):247-253.e1. doi: 10.1016/j.ajo.2013.04.003. Epub 2013 Jun 7. Am J Ophthalmol. 2013. PMID: 23752063
-
Ocular surface injuries in autoimmune dry eye. The severity of microscopical disturbances goes parallel with the severity of symptoms of dryness.Histol Histopathol. 2009 Oct;24(10):1357-65. doi: 10.14670/HH-24.1357. Histol Histopathol. 2009. PMID: 19688700 Review.
-
A Review of the Tear Film Biomarkers Used to Diagnose Sjogren's Syndrome.Int J Mol Sci. 2024 Sep 26;25(19):10380. doi: 10.3390/ijms251910380. Int J Mol Sci. 2024. PMID: 39408709 Free PMC article. Review.
Cited by
-
Protein Signature in Saliva of Temporomandibular Disorders Myalgia.Int J Mol Sci. 2020 Apr 7;21(7):2569. doi: 10.3390/ijms21072569. Int J Mol Sci. 2020. PMID: 32272779 Free PMC article.
-
Role of tear film biomarkers in the diagnosis and management of dry eye disease.Taiwan J Ophthalmol. 2019 Sep 12;9(3):150-159. doi: 10.4103/tjo.tjo_56_19. eCollection 2019 Jul-Sep. Taiwan J Ophthalmol. 2019. PMID: 31572651 Free PMC article. Review.
-
Autoimmune Epithelitis and Chronic Inflammation in Sjögren's Syndrome-Related Dry Eye Disease.Int J Mol Sci. 2021 Oct 30;22(21):11820. doi: 10.3390/ijms222111820. Int J Mol Sci. 2021. PMID: 34769250 Free PMC article. Review.
-
Ion Channels as Potential Drug Targets in Dry Eye Disease and Their Clinical Relevance: A Review.Cells. 2024 Dec 6;13(23):2017. doi: 10.3390/cells13232017. Cells. 2024. PMID: 39682765 Free PMC article. Review.
-
Neutrophil/Lymphocyte Ratio as an Inflammatory Predictor of Dry Eye Disease: A Case-Control Study.Ther Clin Risk Manag. 2021 Mar 23;17:259-266. doi: 10.2147/TCRM.S298156. eCollection 2021. Ther Clin Risk Manag. 2021. PMID: 33790566 Free PMC article.
References
-
- Sjogren H. Zur Kenntnis Der Keratoconjunctivitis Sicca Ii. Acts Opthalmol. 1935;13:1–39. doi: 10.1111/j.1755-3768.1935.tb04186.x. - DOI
-
- Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H., Alexander E., Carsons S., Daniels T., Fox P.C., Fox R., Kassan S.S., et al. Classification criteria for Sjogren syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

