Analysis of the Diffusion Process by pH Indicator in Microfluidic Chips for Liposome Production
- PMID: 30400400
- PMCID: PMC6189829
- DOI: 10.3390/mi8070209
Analysis of the Diffusion Process by pH Indicator in Microfluidic Chips for Liposome Production
Abstract
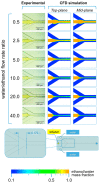
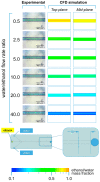
In recent years, the development of nano- and micro-particles has attracted considerable interest from researchers and enterprises, because of the potential utility of such particles as drug delivery vehicles. Amongst the different techniques employed for the production of nanoparticles, microfluidic-based methods have proven to be the most effective for controlling particle size and dispersity, and for achieving high encapsulation efficiency of bioactive compounds. In this study, we specifically focus on the production of liposomes, spherical vesicles formed by a lipid bilayer encapsulating an aqueous core. The formation of liposomes in microfluidic devices is often governed by diffusive mass transfer of chemical species at the liquid interface between a solvent (i.e., alcohol) and a non-solvent (i.e., water). In this work, we developed a new approach for the analysis of mixing processes within microfluidic devices. The method relies on the use of a pH indicator, and we demonstrate its utility by characterizing the transfer of ethanol and water within two different microfluidic architectures. Our approach represents an effective route to experimentally characterize diffusion and advection processes governing the formation of vesicular/micellar systems in microfluidics, and can also be employed to validate the results of numerical modelling.
Keywords: diffusion; liposomes; microfluidic; microfluidic hydrodynamic focusing; mixing; pH indicator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

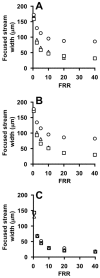
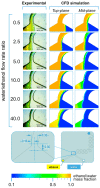
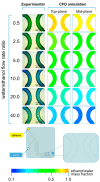
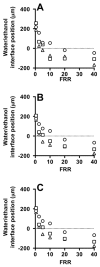
References
-
- Soema P.C., Willems G., Jiskoot W., Amorij J., Kersten G.F. European journal of pharmaceutics and biopharmacutics predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015;94:427–435. doi: 10.1016/j.ejpb.2015.06.026. - DOI - PubMed
-
- Walde P. Preparation of Vesicles (Liposomes) Volume 9 American Scientific Publishers; Valencia, CA, USA: 2004.
LinkOut - more resources
Full Text Sources

