Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications
- PMID: 30400445
- PMCID: PMC6190052
- DOI: 10.3390/mi8080255
Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications
Abstract
Microfluidics is characterized by laminar flow at micro-scale dimension, high surface to volume ratio, and markedly improved heat/mass transfer. In addition, together with advantages of large-scale integration and flexible manipulation, microfluidic technology has been rapidly developed as one of the most important platforms in the field of functional biomaterial synthesis. Compared to biomaterials assisted by conventional strategies, functional biomaterials synthesized by microfluidics are with superior properties and performances, due to their controllable morphology and composition, which have shown great advantages and potential in the field of biomedicine, biosensing, and tissue engineering. Take the significance of microfluidic engineered biomaterials into consideration; this review highlights the microfluidic synthesis technologies and biomedical applications of materials. We divide microfluidic based biomaterials into four kinds. According to the material dimensionality, it includes: 0D (particulate materials), 1D (fibrous materials), 2D (sheet materials), and 3D (construct forms of materials). In particular, micro/nano-particles and micro/nano-fibers are introduced respectively. This classification standard could include all of the microfluidic biomaterials, and we envision introducing a comprehensive and overall evaluation and presentation of microfluidic based biomaterials and their applications.
Keywords: biological applications; controllable synthesis; functional biomaterials; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



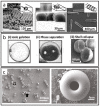





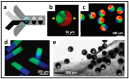

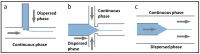

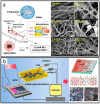



References
-
- Kricka L.J. Miniaturization of analytical systems. Clin. Chem. 1998;44:2008–2014. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

