Anti-Leishmanial and Cytotoxic Activities of a Series of Maleimides: Synthesis, Biological Evaluation and Structure-Activity Relationship
- PMID: 30400596
- PMCID: PMC6278306
- DOI: 10.3390/molecules23112878
Anti-Leishmanial and Cytotoxic Activities of a Series of Maleimides: Synthesis, Biological Evaluation and Structure-Activity Relationship
Abstract
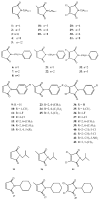
In the present study, 45 maleimides have been synthesized and evaluated for anti-leishmanial activities against L. donovani in vitro and cytotoxicity toward THP1 cells. All compounds exhibited obvious anti-leishmanial activities. Among the tested compounds, there were 10 maleimides with superior anti-leishmanial activities to standard drug amphotericin B, and 32 maleimides with superior anti-leishmanial activities to standard drug pentamidine, especially compounds 16 (IC50 < 0.0128 μg/mL) and 42 (IC50 < 0.0128 μg/mL), which showed extraordinary efficacy in an in vitro test and low cytotoxicities (CC50 > 10 μg/mL). The anti-leishmanial activities of 16 and 42 were 10 times better than that of amphotericin B. The structure and activity relationship (SAR) studies revealed that 3,4-non-substituted maleimides displayed the strongest anti-leishmanial activities compared to those for 3-methyl-maleimides and 3,4-dichloro-maleimides. 3,4-dichloro-maleimides were the least cytotoxic compared to 3-methyl-maleimides and 3,4-non-substituted maleimides. The results show that several of the reported compounds are promising leads for potential anti-leishmanial drug development.
Keywords: Leishmania donovani; SAR; anti-leishmanial activity; cytotoxicity; maleimides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization. [(accessed on 1 September 2017)]; Available online: www.who.int/leishmaniasis/burden/en/
-
- Sharma U., Singh S. Immunobiology of leishmaniasis. Indian J. Exp. Biol. 2009;47:412–423. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

