Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms
- PMID: 30400609
- PMCID: PMC6278502
- DOI: 10.3390/molecules23112881
Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms
Abstract
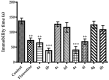
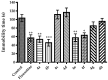
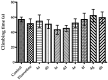
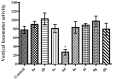
Novel benzazole derivative compounds 4a⁻4h were obtained by the reaction of corresponding 2-(benzazol-2-ylthio)acetohydrazide and appropriate 4-substituted benzaldehydes. The chemical structures of the synthesized compounds were elucidated by FT-IR, ¹H-NMR, 13C-NMR and LCMS spectroscopic methods. Antidepressant-like effects of the compounds were evaluated by tail suspension test (TST) and modified forced swimming tests (MFST). Moreover, locomotor activities of the animals were assessed by an activity cage apparatus. In the series, compounds 4a, 4b, 4e and 4f (at 50 mg/kg) significantly decreased the immobility time of mice in both of the TST and MFST. The same compounds prolonged the swimming time of animals in MFST without any change in the climbing duration. These data indicated that compounds 4a, 4b, 4e and 4f possess significant antidepressant-like activities. Moreover, pre-treatments with p-chloro-phenylalanine methyl ester (an inhibitor of serotonin synthesis), NAN-190 (a 5-HT1A antagonist), ketanserin (a 5-HT2A/2C antagonist), and ondansetron (a 5-HT₃ antagonist) reversed the exhibited pharmacological effects. Results of the mechanistic studies suggested the involvement of serotonergic system and contributions of 5-HT1A, 5-HT2A/2C and 5-HT₃ receptors to the antidepressant-like effects of compounds 4a, 4b, 4e and 4f. Furthermore, unchanged locomotor activity of mice following the administrations of these four derivatives confirmed that the presented antidepressant-like effects are specific.
Keywords: activity cage test; antidepressant; benzazole; modified forced swimming test; tail suspension test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems.Life Sci. 2017 Dec 1;190:110-117. doi: 10.1016/j.lfs.2017.09.023. Epub 2017 Sep 20. Life Sci. 2017. PMID: 28942286
-
Synthesis of Some Novel Thiadiazole Derivative Compounds and Screening Their Antidepressant-Like Activities.Molecules. 2018 Mar 21;23(4):716. doi: 10.3390/molecules23040716. Molecules. 2018. PMID: 29561803 Free PMC article.
-
Synthesis of thiadiazole derivatives bearing hydrazone moieties and evaluation of their pharmacological effects on anxiety, depression, and nociception parameters in mice.Arch Pharm Res. 2012 Mar;35(4):659-69. doi: 10.1007/s12272-012-0410-6. Epub 2012 May 3. Arch Pharm Res. 2012. PMID: 22553059
-
Antidepressant potential of novel flavonoids derivatives from sweet violet (Viola odorata L): Pharmacological, biochemical and computational evidences for possible involvement of serotonergic mechanism.Fitoterapia. 2018 Jul;128:148-161. doi: 10.1016/j.fitote.2018.05.016. Epub 2018 May 24. Fitoterapia. 2018. PMID: 29775777
-
Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice.Prog Neuropsychopharmacol Biol Psychiatry. 2013 Jan 10;40:103-9. doi: 10.1016/j.pnpbp.2012.09.003. Epub 2012 Sep 17. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 22996046
Cited by
-
Design, synthesis, and molecular docking studies of novel pomalidomide-based PROTACs as potential anti-cancer agents targeting EGFRWT and EGFRT790M.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1196-1211. doi: 10.1080/14756366.2022.2062338. J Enzyme Inhib Med Chem. 2022. PMID: 35470756 Free PMC article.
-
Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure-Activity Relationship Studies.Pharmaceutics. 2023 Aug 26;15(9):2208. doi: 10.3390/pharmaceutics15092208. Pharmaceutics. 2023. PMID: 37765177 Free PMC article. Review.
-
Synthesis, Solvatochromism and Estimation of Ground and Excited State Dipole Moments of Silylated Benzothiazole Dyes.J Fluoresc. 2024 Mar;34(2):809-819. doi: 10.1007/s10895-023-03284-2. Epub 2023 Jun 29. J Fluoresc. 2024. PMID: 37382833
-
Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives.Molecules. 2019 May 14;24(10):1857. doi: 10.3390/molecules24101857. Molecules. 2019. PMID: 31091808 Free PMC article.
References
-
- WHO, mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-Specialized Health Settings. [(accessed on 1 May 2018)];2010 Available online: http://apps.who.int/iris/bitstream/10665/44406/1/9789241548069_eng.pdf. - PubMed
-
- WHO, DEPRESSION: A Global Crisis World Mental Health Day. [(accessed on 1 May 2018)];2012 Available online: http://www.who.int/mental_health/management/depression/wfmh_paper_depres....
-
- Yue N., Li B., Yang L., Han Q.Q., Huang H.J., Wang Y.L., Wang J., Yu R., Wu G.C., Liu Q., et al. Electro-Acupuncture Alleviates Chronic Unpredictable Stress-Induced Depressive- and Anxiety-Like Behavior and Hippocampal Neuroinflammation in Rat Model of Depression. Front. Mol. Neurosci. 2018 doi: 10.3389/fnmol.2018.00149. - DOI - PMC - PubMed
-
- Ajani O.O., Tolu-Bolaji O.O., Olorunshola S.J., Zhao Y., Aderohunmu D.V. Structure-based design of functionalized 2-substituted and 1,2-disubstituted benzimidazole derivatives and their in vitro antibacterial efficacy. J. Adv. Res. 2017;8:703–712. doi: 10.1016/j.jare.2017.09.003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

