Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication
- PMID: 30400623
- PMCID: PMC6315955
- DOI: 10.3390/ph11040120
Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication
Abstract
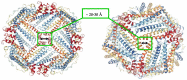
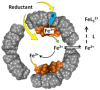
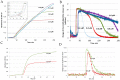
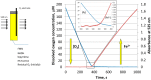
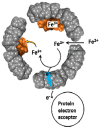
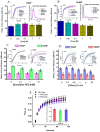
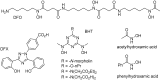
Ferritins are highly conserved supramolecular protein nanostructures composed of two different subunit types, H (heavy) and L (light). The two subunits co-assemble into a 24-subunit heteropolymer, with tissue specific distributions, to form shell-like protein structures within which thousands of iron atoms are stored as a soluble inorganic ferric iron core. In-vitro (or in cell free systems), the mechanisms of iron(II) oxidation and formation of the mineral core have been extensively investigated, although it is still unclear how iron is loaded into the protein in-vivo. In contrast, there is a wide spread belief that the major pathway of iron mobilization from ferritin involves a lysosomal proteolytic degradation of ferritin, and the dissolution of the iron mineral core. However, it is still unclear whether other auxiliary iron mobilization mechanisms, involving physiological reducing agents and/or cellular reductases, contribute to the release of iron from ferritin. In vitro iron mobilization from ferritin can be achieved using different reducing agents, capable of easily reducing the ferritin iron core, to produce soluble ferrous ions that are subsequently chelated by strong iron(II)-chelating agents. Here, we review our current understanding of iron mobilization from ferritin by various reducing agents, and report on recent results from our laboratory, in support of a mechanism that involves a one-electron transfer through the protein shell to the iron mineral core. The physiological significance of the iron reductive mobilization from ferritin by the non-enzymatic FMN/NAD(P)H system is also discussed.
Keywords: chaotropes; electron transfer; ferritin; flavin nucleotide; iron mobilization; kinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
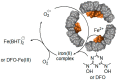
Similar articles
-
Effect of chaotropes on the kinetics of iron release from ferritin by flavin nucleotides.Biochim Biophys Acta Gen Subj. 2017 Dec;1861(12):3257-3262. doi: 10.1016/j.bbagen.2017.09.016. Epub 2017 Sep 21. Biochim Biophys Acta Gen Subj. 2017. PMID: 28943300 Free PMC article.
-
Effect of Phosphate and Ferritin Subunit Composition on the Kinetics, Structure, and Reactivity of the Iron Core in Human Homo- and Heteropolymer Ferritins.Biochemistry. 2022 Oct 4;61(19):2106-2117. doi: 10.1021/acs.biochem.2c00354. Epub 2022 Sep 13. Biochemistry. 2022. PMID: 36099002 Free PMC article.
-
Iron release from ferritin by flavin nucleotides.Biochim Biophys Acta. 2013 Oct;1830(10):4669-74. doi: 10.1016/j.bbagen.2013.05.031. Epub 2013 May 29. Biochim Biophys Acta. 2013. PMID: 23726988
-
Iron mineralization and core dissociation in mammalian homopolymeric H-ferritin: Current understanding and future perspectives.Biochim Biophys Acta Gen Subj. 2020 Nov;1864(11):129700. doi: 10.1016/j.bbagen.2020.129700. Epub 2020 Aug 14. Biochim Biophys Acta Gen Subj. 2020. PMID: 32798636 Review.
-
The ferritins: molecular properties, iron storage function and cellular regulation.Biochim Biophys Acta. 1996 Jul 31;1275(3):161-203. doi: 10.1016/0005-2728(96)00022-9. Biochim Biophys Acta. 1996. PMID: 8695634 Review.
Cited by
-
Examining the Role of a Functional Deficiency of Iron in Lysosomal Storage Disorders with Translational Relevance to Alzheimer's Disease.Cells. 2023 Nov 16;12(22):2641. doi: 10.3390/cells12222641. Cells. 2023. PMID: 37998376 Free PMC article. Review.
-
Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications.Int J Mol Sci. 2022 May 29;23(11):6100. doi: 10.3390/ijms23116100. Int J Mol Sci. 2022. PMID: 35682778 Free PMC article.
-
Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health.Foods. 2024 Jul 17;13(14):2255. doi: 10.3390/foods13142255. Foods. 2024. PMID: 39063339 Free PMC article. Review.
-
Iron Metabolism in the Tumor Microenvironment-Implications for Anti-Cancer Immune Response.Cells. 2021 Feb 2;10(2):303. doi: 10.3390/cells10020303. Cells. 2021. PMID: 33540645 Free PMC article. Review.
-
Probing Subcellular Iron Availability with Genetically Encoded Nitric Oxide Biosensors.Biosensors (Basel). 2022 Oct 21;12(10):903. doi: 10.3390/bios12100903. Biosensors (Basel). 2022. PMID: 36291039 Free PMC article.
References
-
- Cammack R. Iron-Sulfur Clusters in Enzymes-Themes and Variations. Adv. Inorg. Chem. 1992;38:281–322.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

