Sulfatide decreases the resistance to stress-induced apoptosis and increases P-selectin-mediated adhesion: a two-edged sword in breast cancer progression
- PMID: 30400820
- PMCID: PMC6219063
- DOI: 10.1186/s13058-018-1058-z
Sulfatide decreases the resistance to stress-induced apoptosis and increases P-selectin-mediated adhesion: a two-edged sword in breast cancer progression
Abstract
Background: We have previously shown that galactosylceramide (GalCer) affects the tumourigenic and metastatic properties of breast cancer cells by acting as an anti-apoptotic molecule. Since GalCer is a precursor molecule in the synthesis of sulfatides, the present study was aimed to define the role of sulfatides in apoptosis and breast cancer progression.
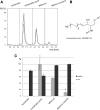
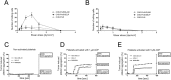
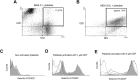
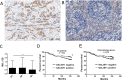
Methods: Expression of GAL3ST1 in breast cancer cell lines and breast cancer tissue specimens was analysed using real-time PCR, western blotting and immunohistochemistry analysis. The amount of sulfatide, GalCer and ceramide was analysed by thin-layer chromatography binding assay and by the modified hydrophilic interaction liquid chromatography coupled with electrospray mass spectrometry methodology. The tumourigenicity of cancer cells was analysed by an in-vivo tumour growth assay. Apoptotic cells were detected based on caspase-3 activation and the TUNEL assay. The interaction of breast cancer cells with P-selectin or E-selectin was analysed using the flow adhesion assay. The ability of sulfatide-expressing cells to activate and aggregate platelets was studied using the flow-cytometry-based aggregation assay.
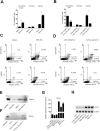
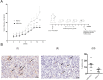
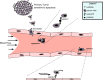
Results: Using two models of breast cancer, T47D cells with blocked synthesis of sulfatide and MDA-MB-231 cells with neosynthesis of this glycosphingolipid, we showed that high sulfatide levels resulted in increased sensitivity of cancer cells to apoptosis induced by hypoxia and doxorubicin in vitro, and decreased their tumourigenicity after transplantation into athymic nu/nu mice. Accordingly, a clinical study on GAL3ST1 expression in invasive ductal carcinoma revealed that its elevated level is associated with better prognosis. Using MDA-MB-231 cells with neosynthesis of sulfatide we also showed that sulfatide is responsible for adhesion of breast cancer cells to P-selectin-expressing cells, including platelets. Sulfatide also acted as an activating molecule, increasing the expression of P-selectin.
Conclusions: This study demonstrates that increased synthesis of sulfatide sensitises cancer cells to microenvironmental stress factors such as hypoxia and anticancer drugs such as doxorubicin. However, sulfatide is probably not directly involved in apoptotic cascades, because its increased synthesis by GAL3ST1 decreased the amounts of its precursor, GalCer, a known anti-apoptotic molecule. On the other hand, our data support the view that sulfatides are malignancy-related adhesive molecules involved in activating and binding P-selectin-expressing platelets to breast cancer cells.
Keywords: Adhesion; Apoptosis; Breast cancer; Galactosyloceramide; P-selectin; Platelets; Sulfatide.
Conflict of interest statement
Ethics approval and consent to participate
The animal study was approved by the Second Local Ethics Committee for Animal Experimentation (reference number: 112/2014, Wroclaw, Poland).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Role for sulfatides in platelet aggregation.Circulation. 2001 Dec 11;104(24):2955-60. doi: 10.1161/hc4901.100383. Circulation. 2001. PMID: 11739312
-
Galactosylceramide affects tumorigenic and metastatic properties of breast cancer cells as an anti-apoptotic molecule.PLoS One. 2013 Dec 31;8(12):e84191. doi: 10.1371/journal.pone.0084191. eCollection 2013. PLoS One. 2013. PMID: 24391908 Free PMC article.
-
Beta-galactosylceramide increases and sulfatide decreases cytokine and chemokine production in whole blood cells.Immunol Lett. 2004 Feb 15;91(2-3):205-11. doi: 10.1016/j.imlet.2003.12.010. Immunol Lett. 2004. PMID: 15019291
-
[The biological role of sulfatides].Postepy Hig Med Dosw (Online). 2016 May 9;70:489-504. doi: 10.5604/17322693.1201720. Postepy Hig Med Dosw (Online). 2016. PMID: 27180966 Review. Polish.
-
Sulfated glycolipids and cell adhesion.Arch Biochem Biophys. 1988 Dec;267(2):405-15. doi: 10.1016/0003-9861(88)90046-x. Arch Biochem Biophys. 1988. PMID: 3063211 Review.
Cited by
-
Cytotoxic capability and the associated proteomic profile of cell-free coelomic fluid extracts from the edible sea cucumber Holothuria tubulosa on HepG2 liver cancer cells.EXCLI J. 2022 Apr 25;21:722-743. doi: 10.17179/excli2022-4825. eCollection 2022. EXCLI J. 2022. PMID: 35721581 Free PMC article.
-
Glycosylation editing: an innovative therapeutic opportunity in precision oncology.Mol Cell Biochem. 2025 Apr;480(4):1951-1967. doi: 10.1007/s11010-024-05033-w. Epub 2024 Jun 11. Mol Cell Biochem. 2025. PMID: 38861100 Review.
-
Tumor ratio of unsaturated to saturated sulfatide species is associated with disease-free survival in intrahepatic cholangiocarcinoma.Cell Oncol (Dordr). 2023 Jun;46(3):629-642. doi: 10.1007/s13402-022-00766-6. Epub 2023 Jan 11. Cell Oncol (Dordr). 2023. PMID: 36630049 Free PMC article.
-
Targeting glucosylceramide synthase induces antiproliferative and proapoptotic effects in osimertinib-resistant NSCLC cell models.Sci Rep. 2024 Mar 18;14(1):6491. doi: 10.1038/s41598-024-57028-8. Sci Rep. 2024. PMID: 38499619 Free PMC article.
-
Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens.Micromachines (Basel). 2022 Oct 19;13(10):1780. doi: 10.3390/mi13101780. Micromachines (Basel). 2022. PMID: 36296133 Free PMC article. Review.
References
-
- Siddiqui B, Whitehead JS, Kim YS. Glycosphingolipids in human colonic adenocarcinoma. J Biol Chem. 1978;253(7):2168–2175. - PubMed
-
- Kiguchi K, Takamatsu K, Tanaka J, Nozawa S, Iwamori M, Nagai Y. Glycosphingolipids of various human ovarian tumors: a significantly high expression of I3SO3GalCer and Lewis antigen in mucinous cystadenocarcinoma. Cancer Res. 1992;52(2):416–421. - PubMed
-
- Liu Y, Chen Y, Momin A, Shaner R, Wang E, Bowen NJ, Matyunina LV, Walker LD, McDonald JF, Sullards MC, et al. Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissue-imaging mass spectrometry. Mol Cancer. 2010;9:186. doi: 10.1186/1476-4598-9-186. - DOI - PMC - PubMed
-
- Sakakibara N, Gasa S, Kamio K, Makita A, Koyanagi T. Association of elevated sulfatides and sulfotransferase activities with human renal cell carcinoma. Cancer Res. 1989;49(2):335–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

