Pragmatic trial of multifaceted intervention (STROKE-CARD care) to reduce cardiovascular risk and improve quality-of-life after ischaemic stroke and transient ischaemic attack -study protocol
- PMID: 30400876
- PMCID: PMC6219064
- DOI: 10.1186/s12883-018-1185-2
Pragmatic trial of multifaceted intervention (STROKE-CARD care) to reduce cardiovascular risk and improve quality-of-life after ischaemic stroke and transient ischaemic attack -study protocol
Abstract
Background: Patients with ischaemic stroke or transient ischaemic attack (TIA) are at high risk of future cardiovascular events. Despite compelling evidence about the efficacy of secondary prevention, a substantial gap exists between risk factor management in real life and that recommended by international guidelines. Moreover, stroke is a leading cause of disability and morbidity which partly emerges from post-stroke complications.
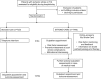
Methods/design: We designed a block-randomised (2:1 ratio) open pragmatic trial [NCT02156778] with blinded outcome assessment comparing STROKE-CARD to usual post-stroke-patient care. STROKE-CARD is a multifaceted post-stroke disease management program with the objective of reducing recurrent cardiovascular events and improving quality of life in ischaemic stroke and TIA-patients. It combines intensified multi-domain secondary prevention, systematic detection and treatment of post-stroke complications, and patient self-empowerment. Enrolment of 2160 patients with acute ischaemic stroke or TIA (ABCD2-Score ≥ 3) is planned at two study centres in Austria. The co-primary efficacy endpoints are (i) the composite of major recurrent cardiovascular events (nonfatal stroke, nonfatal myocardial infarction, and vascular death) occurring within 12 months after the index event and (ii) one-year health-related quality-of-life measured with the European Quality of Life-5 Dimensions (EQ-5D-3 L) questionaire. Secondary endpoints include all-cause mortality, functional outcome, and target-level achievement in risk factor management.
Discussion: This trial will provide evidence on whether the pragmatic post-stroke intervention program STROKE-CARD can help prevent cardiovascular events and improve quality-of-life within the setting of a high-quality acute stroke care system. In case of success, STROKE-CARD may be implemented in daily clinical routine and serve as a model for other disease management initiatives.
Trial registration: ClinicalTrials.gov: NCT02156778 . (June 5, 2014, retrospectively registered).
Keywords: Disease management; Secondary prevention; Stroke; Transient ischemic attack.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the local Ethics Committee of the Medical University of Innsbruck in October 2013 (recruitment start, January 2014) and the local Ethics Committee of the Hospital St. John of God Vienna in December 2014 (recruitment start, December 2014). All patients gave their written informed consent for participation in the trial and the use of bio-samples and data for future research projects targeting etiology, complications, outcome, prognosis, and management of ischaemic stroke and TIA.
Consent for publication
Not applicable.
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Hay SI. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32130-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous