The pervasive effects of recombinant Fasciola gigantica Ras-related protein Rab10 on the functions of goat peripheral blood mononuclear cells
- PMID: 30400957
- PMCID: PMC6219056
- DOI: 10.1186/s13071-018-3148-2
The pervasive effects of recombinant Fasciola gigantica Ras-related protein Rab10 on the functions of goat peripheral blood mononuclear cells
Abstract
Background: Fasciola gigantica-induced immunomodulation is a major hurdle faced by the host for controlling infection. Here, we elucidated the role of F. gigantica Ras-related protein Rab10 (FgRab10) in the modulation of key functions of peripheral blood mononuclear cells (PBMCs) of goats.
Methods: We cloned and expressed recombinant FgRab10 (rFgRab10) protein and examined its effects on several functions of goat PBMCs. Protein interactors of rFgRab10 were predicted in silico by querying the databases Intact, String, BioPlex and BioGrid. In addition, a total energy analysis of each of the identified interactions was also conducted. Gene Ontology (GO) enrichment analysis was carried out using FuncAssociate 3.0.
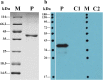
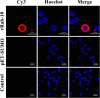
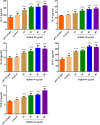
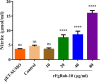
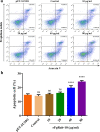
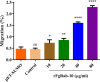
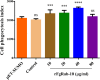
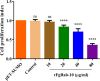
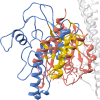
Results: The FgRab10 gene (618 bp), encodes 205-amino-acid residues with a molecular mass of ~23 kDa, had complete nucleotide sequence homology with F. hepatica Ras family protein gene (PIS87503.1). The rFgRab10 protein specifically cross-reacted with anti-Fasciola antibodies as shown by Western blot and immunofluorescence analysis. This protein exhibited multiple effects on goat PBMCs, including increased production of cytokines [interleukin-2 (IL-2), IL-4, IL-10, transforming growth factor beta (TGF-β) and interferon gamma (IFN-γ)] and total nitric oxide (NO), enhancing apoptosis and migration of PBMCs, and promoting the phagocytic ability of monocytes. However, it significantly inhibited cell proliferation. Homology modelling revealed 63% identity between rFgRab10 and human Rab10 protein (Uniprot ID: P61026). Protein interaction network analysis revealed more stabilizing interactions between Rab proteins geranylgeranyltransferase component A 1 (CHM) and Rab proteins geranylgeranyltransferase component A 2 (CHML) and rFgRab10 protein. Gene Ontology analysis identified RabGTPase mediated signaling as the most represented pathway.
Conclusions: rFgRab10 protein exerts profound influences on various functions of goat PBMCs. This finding may help explain why F. gigantica is capable of provoking recognition by host immune cells, less capable of destroying this successful parasite.
Keywords: Fasciola gigantica; Immunomodulation; Peripheral blood mononuclear cells; Ras-related protein Rab10; Recombinant proteins.
Conflict of interest statement
Ethics approval and consent to participate
All experiments in this study were approved by the Science and Technology Agency of Jiangsu Province [Approval number: SYXK (SU) 2010–0005].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
A recombinant Fasciola gigantica 14-3-3 epsilon protein (rFg14-3-3e) modulates various functions of goat peripheral blood mononuclear cells.Parasit Vectors. 2018 Mar 6;11(1):152. doi: 10.1186/s13071-018-2745-4. Parasit Vectors. 2018. PMID: 29510740 Free PMC article.
-
Immune modulation of buffalo peripheral blood mononuclear cells by two asparaginyl endopeptidases from Fasciola gigantica.Parasit Vectors. 2024 Dec 18;17(1):516. doi: 10.1186/s13071-024-06570-5. Parasit Vectors. 2024. PMID: 39696651 Free PMC article.
-
The Multitasking Fasciola gigantica Cathepsin B Interferes With Various Functions of Goat Peripheral Blood Mononuclear Cells in vitro.Front Immunol. 2019 Jul 23;10:1707. doi: 10.3389/fimmu.2019.01707. eCollection 2019. Front Immunol. 2019. PMID: 31396222 Free PMC article.
-
Fasciola hepatica, TGF-β and host mimicry: the enemy within.Curr Opin Microbiol. 2018 Dec;46:80-85. doi: 10.1016/j.mib.2018.09.002. Epub 2018 Oct 11. Curr Opin Microbiol. 2018. PMID: 30317150 Review.
-
Unraveling the microRNAs Involved in Fasciolosis: Master Regulators of the Host-Parasite Crosstalk.Int J Mol Sci. 2024 Dec 29;26(1):204. doi: 10.3390/ijms26010204. Int J Mol Sci. 2024. PMID: 39796061 Free PMC article. Review.
Cited by
-
Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells.Animals (Basel). 2025 Jan 10;15(2):179. doi: 10.3390/ani15020179. Animals (Basel). 2025. PMID: 39858178 Free PMC article.
-
Current Status for Controlling the Overlooked Caprine Fasciolosis.Animals (Basel). 2021 Jun 18;11(6):1819. doi: 10.3390/ani11061819. Animals (Basel). 2021. PMID: 34207215 Free PMC article. Review.
-
Proteomic Profiling of the Liver, Hepatic Lymph Nodes, and Spleen of Buffaloes Infected with Fasciola gigantica.Pathogens. 2020 Nov 24;9(12):982. doi: 10.3390/pathogens9120982. Pathogens. 2020. PMID: 33255373 Free PMC article.
-
Modulation of the Functions of Goat Peripheral Blood Mononuclear Cells by Fasciola gigantica Thioredoxin Peroxidase In Vitro.Pathogens. 2020 Sep 17;9(9):758. doi: 10.3390/pathogens9090758. Pathogens. 2020. PMID: 32957426 Free PMC article.
-
Comparative proteomics of adult Paragonimus kellicotti excretion/secretion products released in vitro or present in the lung cyst nodule.PLoS Negl Trop Dis. 2022 Aug 17;16(8):e0010679. doi: 10.1371/journal.pntd.0010679. eCollection 2022 Aug. PLoS Negl Trop Dis. 2022. PMID: 35976975 Free PMC article.
References
-
- Piedrafita D, Spithill TW, Smith RE, Raadsma HW. Improving animal and human health through understanding liver fluke immunology. Parasite Immunol. 2010;32:572–581. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

