Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial
- PMID: 30400971
- PMCID: PMC6219249
- DOI: 10.1186/s13014-018-1157-0
Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial
Abstract
Background: Cancer cachexia is a prevalent symptom of head and neck neoplasms. The reduction in skeletal muscle mass is one of the main characteristics which can lead to poor physical functioning. The purposes of this pilot randomized controlled trial were to determine the feasibility of progressive resistance training in cachectic head and neck cancer patients during radiotherapy and to explore possible risks and benefits.
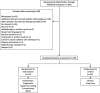
Methods: Twenty cachectic participants with head and neck cancer receiving radiation were randomized to obtain either a machine supported progressive resistance training (n = 10) or usual care (n = 10). The training took place 3 times weekly for 30 min. Intervention included 3 exercises for major muscle groups with 8-12 repetition maximum for 3 sets each. Bioelectrical impedance analysis, hand-held dynamometry, Six-Minute Walk Test and standardized questionnaires for fatigue and quality of life were used for evaluating outcomes at baseline before radiotherapy (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after the end of radiotherapy (t3).
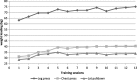
Results: All participants (n = 20) completed the trial. No serious adverse events occurred. At the initial assessment the cachectic patients had already lost 7.1 ± 5.2% of their body weight. General fatigue (score 10.7 ± 3.3) and reduced quality of life (score 71.3 ± 20.6) were prevalent in cachectic head and neck cancer patients even before radiotherapy. An average improvement of weight loading for leg press (+ 19.0%), chest press (+ 29.8%) and latissimus pull-down (+ 22.8%) was possible in the intervention group. Participants had at least 13 training sessions. The outcome measures showed nonsignificant changes at t2 and t3, but a trend for a better course of general fatigue and quality of life at t2 in the intervention group.
Conclusions: Despite advanced tumor stage and burdensome treatment the intervention adherence is excellent. Progressive resistance training in cachectic head and neck cancer patients during radiotherapy seems to be safe and feasible and may have beneficial effects of general fatigue and quality of life.
Trial registration: ClinicalTrials.gov, NCT03524755 . Registered 15 May 2018 - Retrospectively registered.
Keywords: Cachexia; Feasibility study; Head and neck cancer; Radiotherapy; Randomized controlled trial; Resistance training.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: A pilot randomized trial.Cancer. 2017 Nov 15;123(22):4440-4448. doi: 10.1002/cncr.30901. Epub 2017 Jul 31. Cancer. 2017. PMID: 28759113 Clinical Trial.
-
Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy--results from the randomized DAHANCA 25B trial.Radiother Oncol. 2013 Aug;108(2):314-9. doi: 10.1016/j.radonc.2013.07.002. Epub 2013 Aug 6. Radiother Oncol. 2013. PMID: 23932192 Clinical Trial.
-
Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial.Cancer. 2016 Apr 15;122(8):1185-200. doi: 10.1002/cncr.29863. Epub 2016 Feb 1. Cancer. 2016. PMID: 26828426 Clinical Trial.
-
Cachexia and head and neck squamous cell carcinoma: A scoping review.Oral Dis. 2024 May;30(4):1746-1755. doi: 10.1111/odi.14749. Epub 2023 Oct 27. Oral Dis. 2024. PMID: 37891012
-
Body composition changes in patients with head and neck cancer under active treatment: a scoping review.Support Care Cancer. 2020 Oct;28(10):4613-4625. doi: 10.1007/s00520-020-05487-w. Epub 2020 Jun 13. Support Care Cancer. 2020. PMID: 32533436
Cited by
-
Cancer Cachexia: Its Mechanism and Clinical Significance.Int J Mol Sci. 2021 Aug 6;22(16):8491. doi: 10.3390/ijms22168491. Int J Mol Sci. 2021. PMID: 34445197 Free PMC article. Review.
-
The effect of physical exercise during radiotherapy on physical performance in patients with head and neck cancer: a trial within cohorts study protocol, the vital study.BMC Cancer. 2024 Apr 1;24(1):403. doi: 10.1186/s12885-024-12172-2. BMC Cancer. 2024. PMID: 38561708 Free PMC article.
-
Harms of exercise training in patients with cancer undergoing systemic treatment: a systematic review and meta-analysis of published and unpublished controlled trials.EClinicalMedicine. 2023 Apr 6;59:101937. doi: 10.1016/j.eclinm.2023.101937. eCollection 2023 May. EClinicalMedicine. 2023. PMID: 37096190 Free PMC article.
-
Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia.Cancers (Basel). 2024 May 18;16(10):1921. doi: 10.3390/cancers16101921. Cancers (Basel). 2024. PMID: 38791998 Free PMC article. Review.
-
The role of aerobic and resistance exercise for cancer cachexia management - A systematic scoping review.Asia Pac J Oncol Nurs. 2025 Jun 30;12:100748. doi: 10.1016/j.apjon.2025.100748. eCollection 2025 Dec. Asia Pac J Oncol Nurs. 2025. PMID: 40688104 Free PMC article. Review.
References
-
- Stewart BWW, Christopher P. World Cancer Report 2014: Lyon: International Agency for Research on Cancer/world health organization; 2014. 10.1603/EC14197, http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports....
-
- Kaatsch, P., et al., Krebs in Deutschland 2009/2010–9. Ausgabe, 2013. 2013, Robert Koch-Institut.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical