Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment
- PMID: 30401703
- PMCID: PMC6356984
- DOI: 10.1182/blood-2018-04-845420
Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment
Abstract
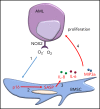
Acute myeloid leukemia (AML) is an age-related disease that is highly dependent on the bone marrow (BM) microenvironment. With increasing age, tissues accumulate senescent cells, characterized by an irreversible arrest of cell proliferation and the secretion of a set of proinflammatory cytokines, chemokines, and growth factors, collectively known as the senescence-associated secretory phenotype (SASP). Here, we report that AML blasts induce a senescent phenotype in the stromal cells within the BM microenvironment and that the BM stromal cell senescence is driven by p16INK4a expression. The p16INK4a-expressing senescent stromal cells then feed back to promote AML blast survival and proliferation via the SASP. Importantly, selective elimination of p16INK4a+ senescent BM stromal cells in vivo improved the survival of mice with leukemia. Next, we find that the leukemia-driven senescent tumor microenvironment is caused by AML-induced NOX2-derived superoxide. Finally, using the p16-3MR mouse model, we show that by targeting NOX2 we reduced BM stromal cell senescence and consequently reduced AML proliferation. Together, these data identify leukemia-generated NOX2-derived superoxide as a driver of protumoral p16INK4a-dependent senescence in BM stromal cells. Our findings reveal the importance of a senescent microenvironment for the pathophysiology of leukemia. These data now open the door to investigate drugs that specifically target the "benign" senescent cells that surround and support AML.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Shafat MS, Oellerich T, Mohr S, et al. . Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129(10):1320-1332. - PubMed
-
- Abdul-Aziz AM, Shafat MS, Mehta TK, et al. . MIF-induced stromal PKCβ/IL8 is essential in human acute myeloid leukemia. Cancer Res. 2017;77(2):303-311. - PubMed
-
- Ryningen A, Wergeland L, Glenjen N, Gjertsen BT, Bruserud O. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leuk Res. 2005;29(2):185-196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

