Anemia of inflammation
- PMID: 30401705
- PMCID: PMC6536698
- DOI: 10.1182/blood-2018-06-856500
Anemia of inflammation
Abstract
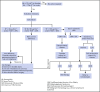
Anemia of inflammation (AI), also known as anemia of chronic disease (ACD), is regarded as the most frequent anemia in hospitalized and chronically ill patients. It is prevalent in patients with diseases that cause prolonged immune activation, including infection, autoimmune diseases, and cancer. More recently, the list has grown to include chronic kidney disease, congestive heart failure, chronic pulmonary diseases, and obesity. Inflammation-inducible cytokines and the master regulator of iron homeostasis, hepcidin, block intestinal iron absorption and cause iron retention in reticuloendothelial cells, resulting in iron-restricted erythropoiesis. In addition, shortened erythrocyte half-life, suppressed erythropoietin response to anemia, and inhibition of erythroid cell differentiation by inflammatory mediators further contribute to AI in a disease-specific pattern. Although the diagnosis of AI is a diagnosis of exclusion and is supported by characteristic alterations in iron homeostasis, hypoferremia, and hyperferritinemia, the diagnosis of AI patients with coexisting iron deficiency is more difficult. In addition to treatment of the disease underlying AI, the combination of iron therapy and erythropoiesis-stimulating agents can improve anemia in many patients. In the future, emerging therapeutics that antagonize hepcidin function and redistribute endogenous iron for erythropoiesis may offer additional options. However, based on experience with anemia treatment in chronic kidney disease, critical illness, and cancer, finding the appropriate indications for the specific treatment of AI will require improved understanding and a balanced consideration of the contribution of anemia to each patient's morbidity and the impact of anemia treatment on the patient's prognosis in a variety of disease settings.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.W. has received lecture honoraria from Vifor. L.T.G. is a consultant for Vifor Pharma and InCube Labs. T.G. is a consultant for Keryx Pharma, Vifor Pharma, Akebia Therapeutics, Gilead Sciences, La Jolla Pharma, and Ionis Pharmaceuticals; has received research funding from Keryx Pharma and Akebia Therapeutics; and is a scientific founder and consultant for Silarus Pharma and Intrinsic LifeSciences.
Figures