Iron metabolism under conditions of ineffective erythropoiesis in β-thalassemia
- PMID: 30401707
- PMCID: PMC6318430
- DOI: 10.1182/blood-2018-07-815928
Iron metabolism under conditions of ineffective erythropoiesis in β-thalassemia
Abstract
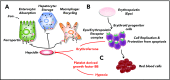
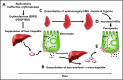
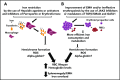
β-Thalassemia (BT) is an inherited genetic disorder that is characterized by ineffective erythropoiesis (IE), leading to anemia and abnormal iron metabolism. IE is an abnormal expansion of the number of erythroid progenitor cells with unproductive synthesis of enucleated erythrocytes, leading to anemia and hypoxia. Anemic patients affected by BT suffer from iron overload, even in the absence of chronic blood transfusion, suggesting the presence of ≥1 erythroid factor with the ability to modulate iron metabolism and dietary iron absorption. Recent studies suggest that decreased erythroid cell differentiation and survival also contribute to IE, aggravating the anemia in BT. Furthermore, hypoxia can also affect and increase iron absorption. Understanding the relationship between iron metabolism and IE could provide important insights into the BT condition and help to develop novel treatments. In fact, genetic or pharmacological manipulations of iron metabolism or erythroid cell differentiation and survival have been shown to improve IE, iron overload, and anemia in animal models of BT. Based on those findings, new therapeutic approaches and drugs have been proposed; clinical trials are underway that have the potential to improve erythrocyte production, as well as to reduce the iron overload and organ toxicity in BT and in other disorders characterized by IE.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: S.R. is a consultant for Ionis Pharmaceuticals, Disc Medicine, Meira GTx, and Protagonist Therapeutics.
Figures
References
-
- Nemeth E, Tuttle MS, Powelson J, et al. . Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

