Mammary Precancerous Stem and Non-Stem Cells Evolve into Cancers of Distinct Subtypes
- PMID: 30401712
- PMCID: PMC6318055
- DOI: 10.1158/0008-5472.CAN-18-1087
Mammary Precancerous Stem and Non-Stem Cells Evolve into Cancers of Distinct Subtypes
Abstract
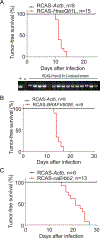
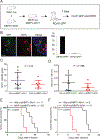
There are distinct cell subpopulations in normal epithelial tissue, including stem cells, progenitor cells, and more differentiated cells, all of which have been extensively studied for their susceptibility to tumorigenesis. However, normal cells usually have to progress through a precancerous lesion state before becoming a full-blown tumor. Precancerous early lesions are heterogeneous, and the cell subset that is the primary source of the eventual tumor remains largely unknown. By using mouse models that are tailored to address this question, we identified a keratin 6a-expressing precancerous stem cell (PcSC) subset and a more differentiated whey acidic protein-positive (WAP+) cell subset in mammary precancerous lesions initiated by the Wnt1 oncogene. Both cell subsets rapidly progressed to cancer upon introduction of constitutively active versions of either HRAS or BRAF. However, the resulting tumors were dramatically different in protein profiles and histopathology: keratin 6a+ precancerous cells gave rise to adenocarcinoma, whereas WAP+ cells yielded metaplastic carcinoma with severe squamous differentiation and more robust activation of MEK/ERK signaling. Therefore, both stem and non-stem cells in mammary precancerous lesions can contribute to the eventual cancers, but their differentiation status determines the resulting cancer phenotype. This work identifies a previously unknown player in cancer heterogeneity and suggests that cancer prevention should target precancerous cells broadly and not be limited to PcSC. SIGNIFICANCE: This work uses a novel mouse mammary gland cancer model to show that tumors initiated from different precancerous mammary epithelial cells are distinct.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
RARα1 control of mammary gland ductal morphogenesis and wnt1-tumorigenesis.Breast Cancer Res. 2010;12(5):R79. doi: 10.1186/bcr2724. Epub 2010 Oct 5. Breast Cancer Res. 2010. PMID: 20923554 Free PMC article.
-
Chromatin effector Pygo2 regulates mammary tumor initiation and heterogeneity in MMTV-Wnt1 mice.Oncogene. 2014 Jan 30;33(5):632-42. doi: 10.1038/onc.2012.620. Epub 2013 Jan 21. Oncogene. 2014. PMID: 23334328 Free PMC article.
-
The transcription factor ATF3 acts as an oncogene in mouse mammary tumorigenesis.BMC Cancer. 2008 Sep 22;8:268. doi: 10.1186/1471-2407-8-268. BMC Cancer. 2008. PMID: 18808719 Free PMC article.
-
Stem/progenitor cells in mouse mammary gland development and breast cancer.J Mammary Gland Biol Neoplasia. 2005 Jan;10(1):17-24. doi: 10.1007/s10911-005-2537-2. J Mammary Gland Biol Neoplasia. 2005. PMID: 15886883 Review.
-
Genetically engineered mouse models of mammary intraepithelial neoplasia.J Mammary Gland Biol Neoplasia. 2000 Oct;5(4):421-37. doi: 10.1023/a:1009534129331. J Mammary Gland Biol Neoplasia. 2000. PMID: 14973386 Review.
Cited by
-
Adjuvant trans-arterial chemoembolization after hepatectomy significantly improves the prognosis of low-risk patients with R0-stage hepatocellular carcinoma.Cancer Manag Res. 2019 May 3;11:4065-4073. doi: 10.2147/CMAR.S195485. eCollection 2019. Cancer Manag Res. 2019. PMID: 31118814 Free PMC article.
-
Napabucasin Attenuates Resistance of Breast Cancer Cells to Tamoxifen by Reducing Stem Cell-Like Properties.Med Sci Monit. 2019 Nov 24;25:8905-8912. doi: 10.12659/MSM.918384. Med Sci Monit. 2019. PMID: 31760402 Free PMC article.
-
Metformin and an insulin/IGF-1 receptor inhibitor are synergistic in blocking growth of triple-negative breast cancer.Breast Cancer Res Treat. 2021 Jan;185(1):73-84. doi: 10.1007/s10549-020-05927-5. Epub 2020 Sep 17. Breast Cancer Res Treat. 2021. PMID: 32940848 Free PMC article.
-
Cell-of-Origin Targeted Drug Repurposing for Triple-Negative and Inflammatory Breast Carcinoma with HDAC and HSP90 Inhibitors Combined with Niclosamide.Cancers (Basel). 2023 Jan 4;15(2):332. doi: 10.3390/cancers15020332. Cancers (Basel). 2023. PMID: 36672285 Free PMC article.
-
ESR1 and p53 interactome alteration defines mechanisms of tamoxifen response in luminal breast cancer.iScience. 2024 May 15;27(6):109995. doi: 10.1016/j.isci.2024.109995. eCollection 2024 Jun 21. iScience. 2024. PMID: 38868185 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

