SNARE-dependent membrane fusion initiates α-granule matrix decondensation in mouse platelets
- PMID: 30401752
- PMCID: PMC6234365
- DOI: 10.1182/bloodadvances.2018019158
SNARE-dependent membrane fusion initiates α-granule matrix decondensation in mouse platelets
Abstract
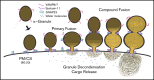
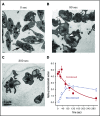
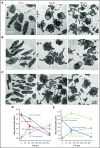
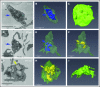
Platelet α-granule cargo release is fundamental to both hemostasis and thrombosis. Granule matrix hydration is a key regulated step in this process, yet its mechanism is poorly understood. In endothelial cells, there is evidence for 2 modes of cargo release: a jack-in-the-box mechanism of hydration-dependent protein phase transitions and an actin-driven granule constriction/extrusion mechanism. The third alternative considered is a prefusion, channel-mediated granule swelling, analogous to the membrane "ballooning" seen in procoagulant platelets. Using thrombin-stimulated platelets from a set of secretion-deficient, soluble N-ethylmaleimide factor attachment protein receptor (SNARE) mutant mice and various ultrastructural approaches, we tested predictions of these mechanisms to distinguish which best explains the α-granule release process. We found that the granule decondensation/hydration required for cargo expulsion was (1) blocked in fusion-protein-deficient platelets; (2) characterized by a fusion-dependent transition in granule size in contrast to a preswollen intermediate; (3) determined spatially with α-granules located close to the plasma membrane (PM) decondensing more readily; (4) propagated from the site of granule fusion; and (5) traced, in 3-dimensional space, to individual granule fusion events at the PM or less commonly at the canalicular system. In sum, the properties of α-granule decondensation/matrix hydration strongly indicate that α-granule cargo expulsion is likely by a jack-in-the-box mechanism rather than by gradual channel-regulated water influx or by a granule-constriction mechanism. These experiments, in providing a structural and mechanistic basis for cargo expulsion, should be informative in understanding the α-granule release reaction in the context of hemostasis and thrombosis.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

