The effects of in ovo administration of encapsulated Toll-like receptor 21 ligand as an adjuvant with Marek's disease vaccine
- PMID: 30401976
- PMCID: PMC6219601
- DOI: 10.1038/s41598-018-34760-6
The effects of in ovo administration of encapsulated Toll-like receptor 21 ligand as an adjuvant with Marek's disease vaccine
Abstract
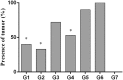
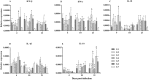
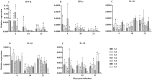
Marek's Disease Virus (MDV) is the causative agent of a lymphoproliferative disease, Marek's disease (MD) in chickens. MD is only controlled by mass vaccination; however, immunity induced by MD vaccines is unable to prevent MDV replication and transmission. The herpesvirus of turkey (HVT) vaccine is one of the most widely used MD vaccines in poultry industry. Vaccines can be adjuvanted with Toll-like receptor ligands (TLR-Ls) to enhance their efficacy. In this study, we examined whether combining TLR-Ls with HVT can boost host immunity against MD and improve its efficacy. Results demonstrated that HVT alone or HVT combined with encapsulated CpG-ODN partially protected chickens from tumor incidence and reduced virus replication compared to the control group. However, encapsulated CpG-ODN only moderately, but not significantly, improved HVT efficacy and reduced tumor incidence from 53% to 33%. Further investigation of cytokine gene profiles in spleen and bursa of Fabricius revealed an inverse association between interleukin (IL)-10 and IL-18 expression and protection conferred by different treatments. In addition, the results of this study raise the possibility that interferon (IFN)-β and IFN-γ induced by the treatments may exert anti-viral responses against MDV replication in the bursa of Fabricius at early stage of MDV infection in chickens.
Conflict of interest statement
The authors declare no competing interests.
Figures

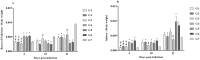
References
-
- Baigent, S. J. & Davison, F. In Marek’s Disease: An Evolving Problem (eds Davison, F. & Nair, V.) 62–77 (Elsevier Academic Press, 2004).
-
- Calnek BW. Lymphomagenesis in Marek’s disease. Avian Pathol. 1998;27:54–64. doi: 10.1080/03079459808419293. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

