Dual mechanisms for the regulation of brain-derived neurotrophic factor by valproic acid in neural progenitor cells
- PMID: 30402028
- PMCID: PMC6205935
- DOI: 10.4196/kjpp.2018.22.6.679
Dual mechanisms for the regulation of brain-derived neurotrophic factor by valproic acid in neural progenitor cells
Erratum in
-
Corrigendum to: Dual mechanisms for the regulation of brain-derived neurotrophic factor by valproic acid in neural progenitor cells.Korean J Physiol Pharmacol. 2019 Jan;23(1):91. doi: 10.4196/kjpp.2019.23.1.91. Epub 2018 Dec 26. Korean J Physiol Pharmacol. 2019. PMID: 30627015 Free PMC article.
Abstract
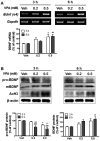
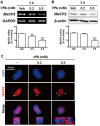
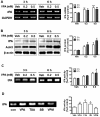
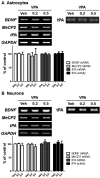
Autism spectrum disorders (ASDs) are neurodevelopmental disorders that share behavioral features, the results of numerous studies have suggested that the underlying causes of ASDs are multifactorial. Behavioral and/or neurobiological analyses of ASDs have been performed extensively using a valid model of prenatal exposure to valproic acid (VPA). Abnormal synapse formation resulting from altered neurite outgrowth in neural progenitor cells (NPCs) during embryonic brain development has been observed in both the VPA model and ASD subjects. Although several mechanisms have been suggested, the actual mechanism underlying enhanced neurite outgrowth remains unclear. In this study, we found that VPA enhanced the expression of brain-derived neurotrophic factor (BDNF), particularly mature BDNF (mBDNF), through dual mechanisms. VPA increased the mRNA and protein expression of BDNF by suppressing the nuclear expression of methyl-CpG-binding protein 2 (MeCP2), which is a transcriptional repressor of BDNF. In addition, VPA promoted the expression and activity of the tissue plasminogen activator (tPA), which induces BDNF maturation through proteolytic cleavage. Trichostatin A and sodium butyrate also enhanced tPA activity, but tPA activity was not induced by valpromide, which is a VPA analog that does not induce histone acetylation, indicating that histone acetylation activity was required for tPA regulation. VPA-mediated regulation of BDNF, MeCP2, and tPA was not observed in astrocytes or neurons. Therefore, these results suggested that VPA-induced mBDNF upregulation was associated with the dysregulation of MeCP2 and tPA in developing cortical NPCs.
Keywords: Brain-derived neurotrophic factor; Methyl-CpG-binding protein 2; Tissue plasminogen activator; Valproic acid.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Figures
References
-
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. - PubMed
-
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. - PubMed
-
- Eadie MJ. Antiepileptic drugs as human teratogens. Expert Opin Drug Saf. 2008;7:195–209. - PubMed
-
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–555. - PubMed
-
- Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. - PubMed

