Sevoflurane Exacerbates Cognitive Impairment Induced by A β 1-40 in Rats through Initiating Neurotoxicity, Neuroinflammation, and Neuronal Apoptosis in Rat Hippocampus
- PMID: 30402039
- PMCID: PMC6198580
- DOI: 10.1155/2018/3802324
Sevoflurane Exacerbates Cognitive Impairment Induced by A β 1-40 in Rats through Initiating Neurotoxicity, Neuroinflammation, and Neuronal Apoptosis in Rat Hippocampus
Abstract
Objective: This study was aimed at investigating whether sevoflurane inhalation induced cognitive impairment in rats with a possible mechanism involved in the event.
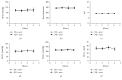
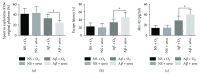
Methods: Thirty-two rats were randomly divided into four groups of normal saline (NS) + O2, NS + sevoflurane (sevo), amyloid-β peptide (Aβ) + O2, and Aβ + sevo. The rats in the four groups received bilateral intrahippocampus injections of NS or Aβ. The treated hippocampus was harvested after inhaling 30% O2 or 2.5% sevoflurane. Evaluation of cognitive function was performed by Morris water maze (MWZ) and an Aβ 1-42 level was determined by ELISA. Protein and mRNA expressions were executed by immunohistochemical (IHC) staining, Western blotting, and qRT-PCR.
Results: Compared with the NS-treated group, sevoflurane only caused cognitive impairment and increased the level of Aβ 1-42 of the brain in the Aβ-treated group. Sevoflurane inhalation but not O2 significantly increased glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule (IBA)1 expression in Aβ-treated hippocampus of rats. Expression levels for Bcl-xL, caspase-9, receptor for advanced glycation end products (RAGE) and brain-derived neurotrophic factor (BDNF) were significantly different in quantification of band intensity between the rats that inhaled O2 and sevoflurane in Aβ-treated groups (all P < 0.05). Interleukin- (IL-) 1β, nuclear factor-κB (NF-κB), and inducible nitric oxide synthase (iNOS) mRNA expression increased after the rats inhaled sevoflurane in the Aβ-treated group (both P < 0.01). There were no significant differences in the change of GFAP, IBA1, Bcl-xL, caspase-9, RAGE, BDNF, IL-1β, NF-κB, and iNOS in the NS + O2 and NS + sevo group (all P > 0.05).
Conclusion: Sevoflurane exacerbates cognitive impairment induced by Aβ 1-40 in rats through initiating neurotoxicity, neuroinflammation, and neuronal apoptosis in rat hippocampus.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

