Sex roles and sexual selection: lessons from a dynamic model system
- PMID: 30402079
- PMCID: PMC6007278
- DOI: 10.1093/cz/zoy036
Sex roles and sexual selection: lessons from a dynamic model system
Abstract
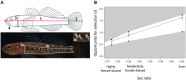
Our understanding of sexual selection has greatly improved during the last decades. The focus is no longer solely on males, but also on how female competition and male mate choice shape ornamentation and other sexually selected traits in females. At the same time, the focus has shifted from documenting sexual selection to exploring variation and spatiotemporal dynamics of sexual selection, and their evolutionary consequences. Here, I review insights from a model system with exceptionally dynamic sexual selection, the two-spotted goby fish Gobiusculus flavescens. The species displays a complete reversal of sex roles over a 3-month breeding season. The reversal is driven by a dramatic change in the operational sex ratio, which is heavily male-biased at the start of the season and heavily female-biased late in the season. Early in the season, breeding-ready males outnumber mature females, causing males to be highly competitive, and leading to sexual selection on males. Late in the season, mating-ready females are in excess, engage more in courtship and aggression than males, and rarely reject mating opportunities. With typically many females simultaneously courting available males late in the season, males become selective and prefer more colorful females. This variable sexual selection regime likely explains why both male and female G. flavescens have ornamental colors. The G. flavescens model system reveals that sexual behavior and sexual selection can be astonishingly dynamic in response to short-term fluctuations in mating competition. Future work should explore whether sexual selection is equally dynamic on a spatial scale, and related spatiotemporal dynamics.
Keywords: Gobiusculus flavescens; OSR; adult sex ratio; female ornament; male ornament; mate choice; mate search; mating competition; operational sex ratio; two-spotted goby.
Figures









Similar articles
-
Unusually dynamic sex roles in a fish.Nature. 2004 Jun 3;429(6991):551-4. doi: 10.1038/nature02562. Nature. 2004. PMID: 15175750
-
Sex roles and mutual mate choice matter during mate sampling.Am Nat. 2012 Jun;179(6):741-55. doi: 10.1086/665651. Epub 2012 Apr 27. Am Nat. 2012. PMID: 22617262
-
Within-season variation in sexual selection in a fish with dynamic sex roles.Mol Ecol. 2014 Jul;23(14):3587-99. doi: 10.1111/mec.12826. Epub 2014 Jun 28. Mol Ecol. 2014. PMID: 24917383
-
The evolution of male mate choice in insects: a synthesis of ideas and evidence.Biol Rev Camb Philos Soc. 2001 Aug;76(3):305-39. doi: 10.1017/s1464793101005693. Biol Rev Camb Philos Soc. 2001. PMID: 11569787 Review.
-
Parental investment, sexual selection and sex ratios.J Evol Biol. 2008 Jul;21(4):919-48. doi: 10.1111/j.1420-9101.2008.01540.x. Epub 2008 May 6. J Evol Biol. 2008. PMID: 18462318 Review.
Cited by
-
Sex Differences in How Territory Quality Affects Aggression in Convict Cichlids.Integr Org Biol. 2021 Oct 18;3(1):obab028. doi: 10.1093/iob/obab028. eCollection 2021. Integr Org Biol. 2021. PMID: 34671717 Free PMC article.
-
Sex roles and the evolution of parental care specialization.Proc Biol Sci. 2019 Aug 28;286(1909):20191312. doi: 10.1098/rspb.2019.1312. Epub 2019 Aug 28. Proc Biol Sci. 2019. PMID: 31455191 Free PMC article.
-
A test of operational sex ratio theory across latitudes reveals temporal variation in sex-specific behavioural reaction norms.J Anim Ecol. 2025 Apr;94(4):642-656. doi: 10.1111/1365-2656.14250. Epub 2025 Feb 18. J Anim Ecol. 2025. PMID: 39967037 Free PMC article.
-
Elevated temperature, but not decreased pH, impairs reproduction in a temperate fish.Sci Rep. 2020 Nov 30;10(1):20805. doi: 10.1038/s41598-020-77906-1. Sci Rep. 2020. PMID: 33257710 Free PMC article.
-
Male mate choice, female competition, and female ornaments as components of sexual selection.Curr Zool. 2018 Jun;64(3):321-322. doi: 10.1093/cz/zoy037. Epub 2018 May 3. Curr Zool. 2018. PMID: 30402074 Free PMC article. No abstract available.
References
-
- Adams S, Mapstone BD, Russ GR, Davies CR, 2000. Geographic variation in the sex ratio, sex specific size, and age structure of Plectropomus leopardus (Serranidae) between reefs open and closed to fishing on the Great Barrier Reef. Can J Fish Aquat Sci 57:1448–1458.
-
- Agorreta A, San Mauro D, Schliewen U, Van Tassell JL, Kovacic M. et al., 2013. Molecular phylogenetics of Gobioidei and phylogenetic placement of European gobies. Mol Phylogenet Evol 69:619–633. - PubMed
-
- Ah-King M, Ahnesjö I, 2013. The “sex role” concept: an overview and evaluation. Evol Biol 40:461–470.
-
- Ahnesjö I, Forsgren E, Kvarnemo C, 2008. Variation in sexual selection in fishes In: Magnhagen C, Braithwaite VB, Forsgren E, Kapoor BG, editors. Fish Behaviour. Boca Ration: CRC Press, 303–335.
-
- Ahnesjö I, Kvarnemo C, Merilaita S, 2001. Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behav Ecol 12:397–401.
LinkOut - more resources
Full Text Sources
Other Literature Sources
