Optimal sampling time-point for cyclosporin A concentration monitoring in heart transplant recipients
- PMID: 30402164
- PMCID: PMC6200973
- DOI: 10.3892/etm.2018.6711
Optimal sampling time-point for cyclosporin A concentration monitoring in heart transplant recipients
Abstract
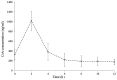
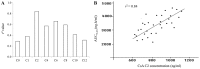
The present study was performed to determine an optimal time-point for monitoring the concentration of the immunosuppressive drug cyclosporin A (CsA) in heart transplant patients and its efficacy in the prevention of transplant rejection. A total of 32 transplant recipients were randomly assigned for three treatment approaches. Recipients in groups A (n=11), B (n=13) and C (n=8) received oral administration of CsA at doses of 3.2, 3.5 and 4.4 mg/kg, respectively. The plasma CsA concentrations were examined at 2 h intervals over 12 h. Furthermore, their correlation with the 4 h pharmacokinetic profiles as the area under the plasma CsA concentration vs. time curve (AUC0-4 h) were calculated The efficacy of CsA in inhibiting cardiac allograft rejection was assessed at 2 h after oral CsA intake (C2) and adverse events of the drug were examined in the C2-monitored recipients. The plasma CsA concentration rapidly increased in most recipients with a peak level detected at ~2 h after dosing. Regression analysis revealed that among all time-points assessed, the CsA had the highest correlation with the AUC0-4 h at C2. At C2, increasing CsA doses exhibited a positive association with the measure of AUC0-4 h. The efficacy of increasing CsA target levels at C2 in preventing heart transplant rejection was comparable, as the survival rate was 100% in all of the treatment groups. However, the proportion of recipients with side effects in group A was obviously lower than that in the other two groups. In conclusion, C2 is an ideal time-point for monitoring plasma CsA levels with a utility for individualising the next scheduled dose for each patient to ensure that target levels are maintained and achieve a high efficacy and safety of CsA therapy in heart transplant recipients (clinical trial no. 12002610).
Keywords: AUC0-4 h; CsA concentration at 2 h; correlation coefficient; cyclosporin A; heart transplantation.
Figures
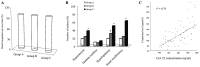
Similar articles
-
Longitudinal evaluation of the pharmacokinetics of cyclosporin microemulsion (Neoral) in pediatric renal transplant recipients and assessment of C2 level as a marker for absorption.Pediatr Transplant. 2003 Aug;7(4):282-8. doi: 10.1034/j.1399-3046.2003.00077.x. Pediatr Transplant. 2003. PMID: 12890006
-
A comparison of measured trough levels and abbreviated AUC estimation by limited sampling strategies for monitoring mycophenolic acid exposure in stable heart transplant patients receiving cyclosporin A-containing and cyclosporin A-free immunosuppressive regimens.Clin Ther. 2006 Jun;28(6):893-905. doi: 10.1016/j.clinthera.2006.06.015. Clin Ther. 2006. PMID: 16860172
-
Limitations of cyclosporine C2 monitoring in pediatric heart transplant recipients.Pediatr Transplant. 2007 Aug;11(5):524-9. doi: 10.1111/j.1399-3046.2007.00712.x. Pediatr Transplant. 2007. PMID: 17631021 Clinical Trial.
-
Distribution of cyclosporin in organ transplant recipients.Clin Pharmacokinet. 2002;41(9):615-37. doi: 10.2165/00003088-200241090-00001. Clin Pharmacokinet. 2002. PMID: 12126456 Review.
-
History of C2 monitoring in heart and liver transplant patients treated with cyclosporine microemulsion.Transplant Proc. 2004 Mar;36(2 Suppl):442S-447S. doi: 10.1016/j.transproceed.2004.01.004. Transplant Proc. 2004. PMID: 15041383 Review.
Cited by
-
Initial dosage optimization of ciclosporin in pediatric Chinese patients who underwent bone marrow transplants based on population pharmacokinetics.Exp Ther Med. 2020 Jul;20(1):401-408. doi: 10.3892/etm.2020.8732. Epub 2020 May 8. Exp Ther Med. 2020. PMID: 32537004 Free PMC article.
-
Optimal single sampling time-point for monitoring of praziquantel exposure in children.Sci Rep. 2021 Sep 9;11(1):17955. doi: 10.1038/s41598-021-97409-x. Sci Rep. 2021. PMID: 34504222 Free PMC article.
-
Tacrolimus and Cyclosporin Pharmacotherapy, Detection Methods, Cytochrome P450 Enzymes after Heart Transplantation.Cardiovasc Hematol Agents Med Chem. 2024;22(2):106-113. doi: 10.2174/1871525721666230726150021. Cardiovasc Hematol Agents Med Chem. 2024. PMID: 37496131 Review.
References
-
- Korewicki J. Cardiac transplantation is still the method of choice in the treatment of patients with severe heart failure. Cardiol J. 2009;16:493–499. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
