Oral Administration of Silk Peptide Enhances the Maturation and Cytolytic Activity of Natural Killer Cells
- PMID: 30402332
- PMCID: PMC6215900
- DOI: 10.4110/in.2018.18.e37
Oral Administration of Silk Peptide Enhances the Maturation and Cytolytic Activity of Natural Killer Cells
Abstract
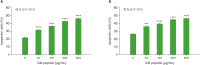
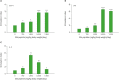
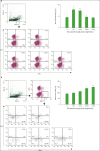
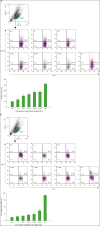
Silk peptide, the hydrolysate of silk protein derived from cocoons, has been employed as a biomedical material and is believed to be safe for human use. Silk peptide display various bioactivities, including anti-inflammatory, immune-regulatory, anti-tumor, anti-viral, and anti-bacterial. Although earlier investigations demonstrated that silk peptide stimulates macrophages and the production of pro-inflammatory cytokines, its effect on natural killer (NK) cell function has not yet been explored. In this study, we initially confirmed that silk peptide enhances NK cell activity in vitro and ex vivo. To assess the modulatory activity of silk peptide on NK cells, mice were fed various amounts of a silk peptide-supplemented diet for 2 months and the effects on immune stimulation, including NK cell activation, were evaluated. Oral administration of silk peptide significantly enhanced the proliferation of mitogen- or IL-2-stimulated splenocytes. In addition, oral silk peptide treatment enhanced the frequency and degree of maturation of NK cells in splenocytes. The same treatment also significantly enhanced the target cell cytolytic activity of NK cells, which was determined by cell surface CD107a expression and intracellular interferon-γ expression. Finally, oral administration of silk peptide stimulated T helper 1-type cytokine expression from splenic lymphocytes. Collectively, our results suggest that silk peptide potentiates NK cell activity in vivo and could be used as a compound for immune-modulating anti-tumor treatment.
Keywords: Cytokine; Cytolytic activity; In vivo; Natural killer cells; Silk peptide.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflict of interest.
Figures
References
-
- Mandal A, Viswanathan C. Natural killer cells: in health and disease. Hematol Oncol Stem Cell Ther. 2015;8:47–55. - PubMed
-
- Moretta L, Ciccone E, Moretta A, Höglund P, Ohlén C, Kärre K. Allorecognition by NK cells: nonself or no self? Immunol Today. 1992;13:300–306. - PubMed
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. - PubMed
-
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. - PubMed
LinkOut - more resources
Full Text Sources

