Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals
- PMID: 30402450
- PMCID: PMC6192081
- DOI: 10.1155/2018/6095097
Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals
Abstract
Background and aims: Chronic hepatitis C is a systemic disease and type 2 diabetes mellitus (T2DM) belongs to more common extrahepatic. The aim of this study was to (i) explore the prevalence of impaired fasting glucose (IFG) and T2DM in patients with chronic hepatitis C, (ii) explore the effect of direct acting antivirals (DAA) treatment on the glycemia, and (iii) explore the factors that modulate the effect of DAA treatment on glycemia in patients with chronic hepatitis C.
Methods: We performed a longitudinal retrospective observational study focused on the patients undergoing DAA treatment of chronic hepatitis C. Data about glycemia, history of diabetes, hepatitis C virus, treatment, and liver status, including elastography, were obtained at baseline (before treatment start), at the end of treatment and 12 weeks after the end of treatment. Patients were treated with various regimens of direct acting antivirals.
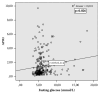
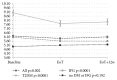
Results: We included 370 patients; 45.9% had F4 fibrosis. At baseline, the prevalence of T2DM increased with the degree of fibrosis (F0-F2 14.4%, F3 21.3%, and F4 31.8%, p=0.004). Fasting glycemia also increased with the degree of fibrosis (F0-F2 5.75±0.18 F3 5.84±0.17, and F4 6.69±0.2 mmol/L, p=0.001). We saw significant decrease of glycemia after treatment in all patients, but patients without T2DM or IFG from 6.21±0.12 to 6.08±0.15 mmol/L (p=0.002). The decrease was also visible in treatment experienced patients and patients with Child-Pugh A cirrhosis.
Conclusion: We confirmed that the prevalence of either T2DM or IFG increases in chronic hepatitis C patients with the degree of fibrosis. The predictive factors for T2DM were, besides F4, fibrosis also higher age and BMI. Significant decrease of fasting glycemia after the DAA treatment was observed in the whole cohort and in subgroups of patients with T2DM, IFG, cirrhotic, and treatment experienced patients.
Figures



Similar articles
-
Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study.Am J Gastroenterol. 2008 Oct;103(10):2481-7. doi: 10.1111/j.1572-0241.2008.02002.x. Epub 2008 Aug 8. Am J Gastroenterol. 2008. PMID: 18702647 Clinical Trial.
-
Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection.AIDS. 2018 Oct 23;32(16):2347-2352. doi: 10.1097/QAD.0000000000001966. AIDS. 2018. PMID: 30096074
-
Impact of direct acting antiviral (DAA) treatment on glucose metabolism and reduction of pre-diabetes in patients with chronic hepatitis C.J Gastrointestin Liver Dis. 2018 Sep;27(3):281-289. doi: 10.15403/jgld.2014.1121.273.daa. J Gastrointestin Liver Dis. 2018. PMID: 30240472
-
Chronic Hepatitis C Association with Diabetes Mellitus and Cardiovascular Risk in the Era of DAA Therapy.Can J Gastroenterol Hepatol. 2018 Aug 13;2018:6150861. doi: 10.1155/2018/6150861. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 30186821 Free PMC article. Review.
-
Extrahepatic manifestations of HCV: the role of direct acting antivirals.Expert Rev Anti Infect Ther. 2017 Aug;15(8):737-746. doi: 10.1080/14787210.2017.1354697. Epub 2017 Jul 17. Expert Rev Anti Infect Ther. 2017. PMID: 28696154 Review.
Cited by
-
The Effect of Viral Clearance Achieved by Direct-Acting Antiviral Agents on Hepatitis C Virus Positive Patients with Type 2 Diabetes Mellitus: A Word of Caution after the Initial Enthusiasm.J Clin Med. 2020 Feb 19;9(2):563. doi: 10.3390/jcm9020563. J Clin Med. 2020. PMID: 32092892 Free PMC article. Review.
-
Natural History of Hepatic and Extrahepatic Hepatitis C Virus Diseases and Impact of Interferon-Free HCV Therapy.Cold Spring Harb Perspect Med. 2020 Apr 1;10(4):a036921. doi: 10.1101/cshperspect.a036921. Cold Spring Harb Perspect Med. 2020. PMID: 31636094 Free PMC article. Review.
-
Effects of hepatitis C virus genotypes and viral load on glucose and lipid metabolism after sustained virological response with direct-acting antivirals.Rev Assoc Med Bras (1992). 2023 May 19;69(5):e20221163. doi: 10.1590/1806-9282.20221163. eCollection 2023. Rev Assoc Med Bras (1992). 2023. PMID: 37222317 Free PMC article.
-
AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study.Life (Basel). 2023 Sep 7;13(9):1881. doi: 10.3390/life13091881. Life (Basel). 2023. PMID: 37763285 Free PMC article.
-
Increased cardiovascular risk and reduced quality of life are highly prevalent among individuals with hepatitis C.BMJ Open Gastroenterol. 2020 Aug;7(1):e000470. doi: 10.1136/bmjgast-2020-000470. BMJ Open Gastroenterol. 2020. PMID: 32847899 Free PMC article.
References
-
- van der Meer A. J., Veldt B. J., Feld J. J., et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. The Journal of the American Medical Association. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

