Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface
- PMID: 30402466
- PMCID: PMC6193326
- DOI: 10.1155/2018/2758347
Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface
Abstract
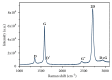
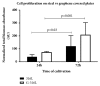
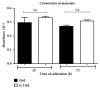
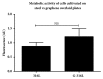
Stainless steel 316L is a material commonly used in cardiovascular medicine. Despite the various methods applied in stent production, the rates of in-stent restenosis and thrombosis remain high. In this study graphene was used to coat the surface of 316L substrate for enhanced bio- and hemocompatibility of the substrate. The presence of graphene layers applied to the substrate was investigated using cutting-edge imaging technology: energy-filtered low-voltage FE-SEM approach, scanning electron microscopy (SEM), Raman spectroscopy, and atomic force microscopy (AFM). The potential of G-316L surface to influence endothelial cells phenotype and endothelial-to-mesenchymal transition (EndoMT) has been determined. Our results show that the bio- and hemocompatible properties of graphene coatings along with known radial force of 316L make G-316L a promising candidate for intracoronary implants.
Figures




Similar articles
-
Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents.Anal Bioanal Chem. 2013 Jan;405(1):321-9. doi: 10.1007/s00216-012-6449-x. Epub 2012 Oct 9. Anal Bioanal Chem. 2013. PMID: 23052887
-
Covalent immobilization of biomolecules on stent materials through mussel adhesive protein coating to form biofunctional films.Mater Sci Eng C Mater Biol Appl. 2020 Jan;106:110187. doi: 10.1016/j.msec.2019.110187. Epub 2019 Sep 10. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31753395
-
Innovative coating based on graphene and their decorated nanoparticles for medical stent applications.Mater Sci Eng C Mater Biol Appl. 2019 Mar;96:708-715. doi: 10.1016/j.msec.2018.11.084. Epub 2018 Dec 4. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30606584
-
Role of stent design and coatings on restenosis and thrombosis.Adv Drug Deliv Rev. 2006 Jun 3;58(3):377-86. doi: 10.1016/j.addr.2006.01.022. Epub 2006 Mar 6. Adv Drug Deliv Rev. 2006. PMID: 16650911 Review.
-
Bio-Based Covered Stents: The Potential of Biologically Derived Membranes.Tissue Eng Part B Rev. 2019 Apr;25(2):135-151. doi: 10.1089/ten.TEB.2018.0207. Tissue Eng Part B Rev. 2019. PMID: 30311858 Review.
Cited by
-
Graphene Coating Obtained in a Cold-Wall CVD Process on the Co-Cr Alloy (L-605) for Medical Applications.Int J Mol Sci. 2021 Mar 13;22(6):2917. doi: 10.3390/ijms22062917. Int J Mol Sci. 2021. PMID: 33805752 Free PMC article.
-
Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials.Materials (Basel). 2020 Apr 23;13(8):1980. doi: 10.3390/ma13081980. Materials (Basel). 2020. PMID: 32340390 Free PMC article.
-
Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel.Materials (Basel). 2021 Apr 30;14(9):2342. doi: 10.3390/ma14092342. Materials (Basel). 2021. PMID: 33946387 Free PMC article.
-
Impact of Magnetite Nanowires on In Vitro Hippocampal Neural Networks.Biomolecules. 2023 Apr 30;13(5):783. doi: 10.3390/biom13050783. Biomolecules. 2023. PMID: 37238653 Free PMC article.
-
Functional Mechanical Behavior and Biocompatible Characteristics of Graphene-Coated Cardiovascular Stents.Int J Mol Sci. 2024 Dec 12;25(24):13345. doi: 10.3390/ijms252413345. Int J Mol Sci. 2024. PMID: 39769110 Free PMC article.
References
-
- Antman E. M., Anbe D. T., Armstrong P. W., et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Journal of the American College of Cardiology. 2004;44(3):671–719. doi: 10.1016/j.jacc.2004.07.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

