Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit
- PMID: 30402473
- PMCID: PMC6196994
- DOI: 10.1155/2018/3623274
Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit
Abstract
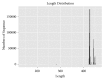
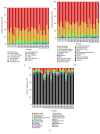
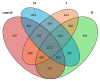
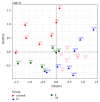
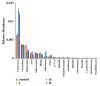
Mycotoxins exhibit several severe effects on intestinal health, but few studies have assessed mycotoxins effect on the intestinal microflora and its repercussions to humans and animals. In this study, we evaluated the effect of zearalenone (ZEA), one of the most harmful mycotoxins on the structure of caecal microbiota in rabbits. Twenty-eight male weaned rabbits were randomly divided into four groups and orally given different concentrations of ZEA (400, 800, and 1600 μg/kg.b.w). Microbial communities in caecum samples of rabbits were analyzed for 16S rRNA by Illumina sequencing through Illumina Miseq platform after being fed for 28 days. The results showed that increasing ZEA doses increased the species richness but did not significantly increased the species diversity of the caecum microbiota in the rabbits. In addition, the caecum microbiota from the samples in different ZEA-treated groups was clustered according to their dosing regimens. At the phylum level, ZEA decreased the abundance of Actinobacteria and significantly increased the abundance of Cyanobacteria, Synergistetes, and Proteobacteria. At the genus level, there were declines in the abundance of Adlercreutzia, Blautia, Desulfitobacter, Lactobacillus, Oxalobacter, and p-75-a5. The decrease of abundance in Lactobacillus, Desulfitobacter, and p-75-a5 was particularly noticeable. In conclusion, zearalenone could increase α-diversity but significantly decrease the abundance of some bacteria with the important metabolic functions. These findings suggested that ZEA could modify the caecum microbiota.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

