Excess Hydrocortisone Hampers Placental Nutrient Uptake Disrupting Cellular Metabolism
- PMID: 30402483
- PMCID: PMC6198558
- DOI: 10.1155/2018/5106174
Excess Hydrocortisone Hampers Placental Nutrient Uptake Disrupting Cellular Metabolism
Abstract
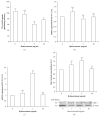
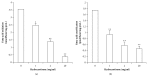
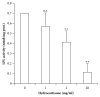
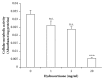
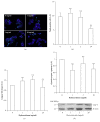
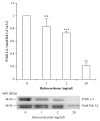
Low birth weight increases neonatal morbidity and mortality, and surviving infants have increased risk of metabolic and cardiovascular disturbances later in life, as well as other neurological, psychiatric, and immune complications. A gestational excess of glucocorticoids (GCs) is a well-known cause for fetal growth retardation, but the biological basis for this association remains elusive. Placental growth is closely related to fetal growth. The placenta is the main regulator of nutrient transport to the fetus, resulting from the difference between placental nutrient uptake and the placenta's own metabolism. The aim of this study was to analyze how excess hydrocortisone affects placental glucose and lipid metabolism. Human placenta explants from term physiological pregnancies were cultured for 18 hours under different hydrocortisone concentrations (2.75, 5.5, and 55 mM; 1, 2, and 20 mg/ml). Placental glucose and lipid uptake and the metabolic partitioning of fatty acids were quantified by isotopic techniques, and expression of specific glucose transporter GLUT1 was quantified by western blot. Cell viability was assessed by MTT, immunohistochemistry and caspase activity. We found that excess hydrocortisone impairs glucose uptake and lipoprotein lipase (LPL) activity, coincident with a GC-dose dependent inhibition of fatty acid oxidation and esterification. None of the experimental conditions showed an increased cell death. In conclusion, our results show that GC overexposure exerts a dysfunctional effect on lipid transport and metabolism and glucose uptake in human placental explants. These findings could well be directly related to a reduced placental growth and possibly to a reduced supply of nutrients to the fetus and the consequent fetal growth retardation and metabolic programming.
Figures