TNIP1 in Autoimmune Diseases: Regulation of Toll-like Receptor Signaling
- PMID: 30402506
- PMCID: PMC6192141
- DOI: 10.1155/2018/3491269
TNIP1 in Autoimmune Diseases: Regulation of Toll-like Receptor Signaling
Abstract
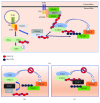
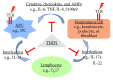
TNIP1 protein is increasingly being recognized as a key repressor of inflammatory signaling and a potential factor in multiple autoimmune diseases. In addition to earlier foundational reports of TNIP1 SNPs in human autoimmune diseases and TNIP1 protein-protein interaction with receptor regulating proteins, more recent studies have identified new potential interaction partners and signaling pathways likely modulated by TNIP1. Subdomains within the TNIP1 protein as well as how they interact with ubiquitin have not only been mapped but inflammatory cell- and tissue-specific consequences subsequent to their defective function are being recognized and related to human disease states such as lupus, scleroderma, and psoriasis. In this review, we emphasize receptor signaling complexes and regulation of cytoplasmic signaling steps downstream of TLR given their association with some of the same autoimmune diseases where TNIP1 has been implicated. TNIP1 dysfunction or deficiency may predispose healthy cells to the inflammatory response to otherwise innocuous TLR ligand exposure. The recognition of the anti-inflammatory roles of TNIP1 and improved integrated understanding of its physical and functional association with other signaling pathway proteins may position TNIP1 as a candidate target for the design and/or testing of next-generation anti-inflammatory therapeutics.
Figures
Similar articles
-
TNIP1 reduction sensitizes keratinocytes to post-receptor signalling following exposure to TLR agonists.Cell Signal. 2018 May;45:81-92. doi: 10.1016/j.cellsig.2018.02.004. Epub 2018 Feb 5. Cell Signal. 2018. PMID: 29413846
-
Emerging roles for TNIP1 in regulating post-receptor signaling.Cytokine Growth Factor Rev. 2012 Jun;23(3):109-18. doi: 10.1016/j.cytogfr.2012.04.002. Epub 2012 Apr 28. Cytokine Growth Factor Rev. 2012. PMID: 22542476 Free PMC article. Review.
-
Downregulation of TNIP1 Expression Leads to Increased Proliferation of Human Keratinocytes and Severer Psoriasis-Like Conditions in an Imiquimod-Induced Mouse Model of Dermatitis.PLoS One. 2015 Jun 5;10(6):e0127957. doi: 10.1371/journal.pone.0127957. eCollection 2015. PLoS One. 2015. PMID: 26046540 Free PMC article.
-
Enhanced Wound Healing- and Inflammasome-Associated Gene Expression in TNFAIP3-Interacting Protein 1- (TNIP1-) Deficient HaCaT Keratinocytes Parallels Reduced Reepithelialization.Mediators Inflamm. 2020 Apr 21;2020:5919150. doi: 10.1155/2020/5919150. eCollection 2020. Mediators Inflamm. 2020. PMID: 32377162 Free PMC article.
-
Toll-Like Receptor Pathways in Autoimmune Diseases.Clin Rev Allergy Immunol. 2016 Feb;50(1):1-17. doi: 10.1007/s12016-015-8473-z. Clin Rev Allergy Immunol. 2016. PMID: 25687121 Review.
Cited by
-
Complexities in Genetics of Psoriatic Arthritis.Curr Rheumatol Rep. 2020 Mar 12;22(4):10. doi: 10.1007/s11926-020-0886-x. Curr Rheumatol Rep. 2020. PMID: 32166449 Free PMC article. Review.
-
CCL3L3-null status is associated with susceptibility to systemic lupus erythematosus.Sci Rep. 2021 Sep 27;11(1):19172. doi: 10.1038/s41598-021-98531-6. Sci Rep. 2021. PMID: 34580371 Free PMC article.
-
Immunogenetics of Systemic Sclerosis.Genes (Basel). 2024 May 5;15(5):586. doi: 10.3390/genes15050586. Genes (Basel). 2024. PMID: 38790215 Free PMC article. Review.
-
Autoimmunity in psoriatic arthritis: pathophysiological and clinical aspects.Turk J Med Sci. 2021 Aug 30;51(4):1601-1614. doi: 10.3906/sag-2011-235. Turk J Med Sci. 2021. PMID: 33581710 Free PMC article. Review.
-
Construction and analysis for dys-regulated lncRNAs and mRNAs in LPS-induced porcine PBMCs.Innate Immun. 2021 Feb;27(2):170-183. doi: 10.1177/1753425920983869. Epub 2021 Jan 27. Innate Immun. 2021. PMID: 33504244 Free PMC article.
References
-
- US Department of Health and Human Services. NIH progress in autoimmune diseases research. National Institutes of Health; 2005. Publication No. 05-514.
-
- Lerner A., Jeremias P., Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. International Journal of Celiac Disease. 2015;3(4):151–155. doi: 10.12691/ijcd-3-4-8. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

