Randomized controlled trial to evaluate locally sourced two-component compression bandages for HIV-associated Kaposi sarcoma leg lymphedema in western Kenya: The Kenyan Improvised Compression for Kaposi Sarcoma (KICKS) study protocol
- PMID: 30402565
- PMCID: PMC6205322
- DOI: 10.1016/j.conctc.2018.10.003
Randomized controlled trial to evaluate locally sourced two-component compression bandages for HIV-associated Kaposi sarcoma leg lymphedema in western Kenya: The Kenyan Improvised Compression for Kaposi Sarcoma (KICKS) study protocol
Abstract
Background: HIV-associated Kaposi sarcoma (KS), among the most frequent cancers seen in sub-Saharan Africa, is associated with a high prevalence of lymphedema. Lymphedema causes progressive functional impairment marked by swelling, physical discomfort, disfiguring changes, skin hardening from fibrosis, poor wound healing, and recurrent skin infection. While compression therapy is considered a major component of lymphedema management, this intervention has never been evaluated in HIV-associated KS lymphedema.
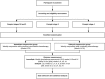
Methods/design: The Kenyan Improvised Compression for Kaposi Sarcoma (KICKS) study is a randomized, controlled trial. Due to variable lymphedema stage, we will use block randomization with a 1:1 allocation to assign participants to one of two groups: "Immediate compression" or "Delayed compression." Those randomized to "Immediate compression" intervention arm will receive weekly two-component compression bandages while receiving chemotherapy, whereas those in the "Delayed compression" control arm will be followed during chemotherapy and then receive compression after chemotherapy is completed. The primary outcome is change in Lower Extremity Lymphedema Index from enrollment at Week 0 to blinded outcome assessment at Week 14 between intervention and control arms. Secondary outcomes are change in leg lymphedema-specific quality of life (LYMQOL) and change in overall health quality of life in cancer (EORTC QLQ C30).
Discussion: This represents the first study in sub-Saharan Africa to assess a lymphedema-directed intervention for KS, and the intervention-locally sourced two-component compression bandages-is affordable and available. Thus, the KICKS study is an important step towards developing an evidence-based path for regionally relevant management of HIV-associated KS lymphedema.
Trial registration: This trial was registered at ClinicalTrials.gov on January 19, 2018: identifier NCT03404297.
Keywords: Compression; Kaposi sarcoma; Lymphedema; Paste bandage; Randomized controlled trial; Unna boot.
Similar articles
-
Compression Therapy for HIV-Associated Kaposi Sarcoma Leg Lymphedema: Results of the Kenyan Improvised Compression for Kaposi Sarcoma Randomized Controlled Trial.JCO Glob Oncol. 2022 Jan;8:e2100329. doi: 10.1200/GO.21.00329. JCO Glob Oncol. 2022. PMID: 35025687 Free PMC article. Clinical Trial.
-
Implementing a Locally Made Low-Cost Intervention for Wound and Lymphedema Care in Western Kenya.Dermatol Clin. 2021 Jan;39(1):91-100. doi: 10.1016/j.det.2020.08.009. Epub 2020 Oct 31. Dermatol Clin. 2021. PMID: 33228865 Free PMC article.
-
Development of Low-Cost Locally Sourced Two-Component Compression Bandages in Western Kenya.Dermatol Ther (Heidelb). 2018 Sep;8(3):475-481. doi: 10.1007/s13555-018-0248-z. Epub 2018 Jun 15. Dermatol Ther (Heidelb). 2018. PMID: 29905913 Free PMC article.
-
Lymphedema and Kaposi sarcoma: A narrative review.J Med Vasc. 2023 Nov-Dec;48(5-6):181-187. doi: 10.1016/j.jdmv.2023.10.007. Epub 2023 Nov 8. J Med Vasc. 2023. PMID: 38035924 Review.
-
Oral HIV-associated Kaposi sarcoma.J Oral Pathol Med. 2013 Mar;42(3):201-7. doi: 10.1111/j.1600-0714.2012.01180.x. Epub 2012 Jun 5. J Oral Pathol Med. 2013. PMID: 22672182 Review.
Cited by
-
A Global Health Reciprocal Innovation grant programme: 5-year review with lessons learnt.BMJ Glob Health. 2023 Nov;8(Suppl 7):e013585. doi: 10.1136/bmjgh-2023-013585. BMJ Glob Health. 2023. PMID: 37977589 Free PMC article.
-
Compression Therapy for HIV-Associated Kaposi Sarcoma Leg Lymphedema: Results of the Kenyan Improvised Compression for Kaposi Sarcoma Randomized Controlled Trial.JCO Glob Oncol. 2022 Jan;8:e2100329. doi: 10.1200/GO.21.00329. JCO Glob Oncol. 2022. PMID: 35025687 Free PMC article. Clinical Trial.
-
Implementing a Locally Made Low-Cost Intervention for Wound and Lymphedema Care in Western Kenya.Dermatol Clin. 2021 Jan;39(1):91-100. doi: 10.1016/j.det.2020.08.009. Epub 2020 Oct 31. Dermatol Clin. 2021. PMID: 33228865 Free PMC article.
-
Engaging Patients for Clinical Trials in Africa: Patient-Centered Approaches.JCO Glob Oncol. 2020 Jun;6:942-947. doi: 10.1200/JGO.19.00190. JCO Glob Oncol. 2020. PMID: 32614725 Free PMC article.
References
-
- Rohner E., Valeri F., Maskew M., Prozesky H., Rabie H., Garone D., Dickinson D., Chimbetete C., Lumano-Mulenga P., Sikazwe I. Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: a prospective multicohort study. J. Acquir. Immune Defic. Syndr. 2014;67(5):547–554. - PMC - PubMed