Avoiding contact allergens: from basic research to the in vitro identification of contact allergens
- PMID: 30402606
- PMCID: PMC6039996
- DOI: 10.5414/ALX01440E
Avoiding contact allergens: from basic research to the in vitro identification of contact allergens
Abstract
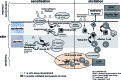
Allergic contact dermatitis (ACD) is a chemical-induced inflammatory skin disease. Contact allergens are low-molecular-weight chemicals that must react with proteins in order to become immunogenic. This interaction leads to the activation of innate immune and stress responses and to the formation of antigenic epitopes for T cells which are the effector cells of ACD. Due to the multitude of chemicals that surround us in our daily life and their potential sensitizing capacity, it is crucial to identify contact sensitizers before these chemicals are used in consumer products. Appropriate in vitro assays for hazard identification are urgently needed to replace animal-based assays. The EU-wide ban on sensitization testing of cosmetic ingredients in animals is in effect since March 2009 and the necessity to test more than 30,000 already marketed chemicals for their sensitizing potential under the EU regulation REACh has intensified the worldwide efforts to replace animal testing. We summarize here the current strategies to develop a battery of assays which allows the identification of contact allergens by in vitro alternatives to animal testing. Our main focus lies on the test systems recently developed within the EU project Sens-it-iv in which we participate.
Keywords: contact dermatitis; in vitro assay; inflammation; innate immune system; skin.
Figures

Republished from
- pp. 529-537
Similar articles
-
The role of the innate immune system in allergic contact dermatitis.Allergol Select. 2017 Aug 4;1(1):39-43. doi: 10.5414/ALX01274E. eCollection 2017. Allergol Select. 2017. PMID: 30402600 Free PMC article. Review.
-
An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing.Cutan Ocul Toxicol. 2012 Dec;31(4):292-305. doi: 10.3109/15569527.2012.667031. Epub 2012 Apr 12. Cutan Ocul Toxicol. 2012. PMID: 22494060
-
Skin sensitizers in cosmetics and beyond: potential multiple mechanisms of action and importance of T-cell assays for in vitro screening.Crit Rev Toxicol. 2017 May;47(5):415-432. doi: 10.1080/10408444.2017.1288025. Epub 2017 Mar 22. Crit Rev Toxicol. 2017. PMID: 28326907 Review.
-
T-cell recognition of chemicals, protein allergens and drugs: towards the development of in vitro assays.Cell Mol Life Sci. 2010 Dec;67(24):4171-84. doi: 10.1007/s00018-010-0495-3. Epub 2010 Aug 18. Cell Mol Life Sci. 2010. PMID: 20717835 Free PMC article. Review.
-
In vitro measurement of skin sensitization hazard of mixtures and finished products: Results obtained with the SENS-IS assays.Toxicol In Vitro. 2020 Feb;62:104644. doi: 10.1016/j.tiv.2019.104644. Epub 2019 Sep 10. Toxicol In Vitro. 2020. PMID: 31518668
Cited by
-
Viability of cultured human skin cells treated with 1,6-hexamethylene diisocyanate monomer and its oligomer isocyanurate in different culture media.Sci Rep. 2021 Dec 10;11(1):23804. doi: 10.1038/s41598-021-02811-0. Sci Rep. 2021. PMID: 34893638 Free PMC article.
References
-
- Rouvier E Luciani MF Mattéi MG Denizot F Golstein P CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993; 150: 5445–5456. - PubMed
-
- Dumoutier L Van Roost E Ameye G Michaux L Renauld JC IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 2000; 1: 488–494. - PubMed
-
- Weaver CT Harrington LE Mangan PR Gavrieli M Murphy KM Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006; 24: 677–688. - PubMed
-
- Schmidt-Weber CB Akdis M Akdis CA TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007; 120: 247–254. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
