Epidemiology of cutaneous adverse drug reactions
- PMID: 30402608
- PMCID: PMC6039997
- DOI: 10.5414/ALX01508E
Epidemiology of cutaneous adverse drug reactions
Abstract
Epidemiologic investigation of cutaneous adverse drug reactions (cADRs) is important in order to evaluate their impact on dermatology and health care in general as well as their burden on affected patients. Few epidemiologic studies have been performed on frequent non-life-threatening cADR, including reactions of both delayed and immediate hypersensitivity, such as maculopapular exanthema (MPE), fixed drug eruption, and urticaria. Concerning rare but life-threatening severe cutaneous adverse reactions, e.g., toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS), several epidemiologic studies have been performed to date, some of which are still ongoing. Such studies enable the calculation of reliable incidence rates and demographic data, and also allow researchers to perform risk estimation for drugs. The spectrum of drugs causing cADR differs substantially when separating the various clinical conditions. Whereas antibiotics are by far the most frequent inducers of milder cADRs, like MPE, they have a much lower risk of inducing SJS/TEN, for which "high-risk" drugs are anti-infective sulfonamides, allopurinol, certain anti-epileptic drugs, nevirapine, and non-steroidal anti-inflammatory drugs (NSAIDs) of the oxicam-type. In contrast, AGEP is predominantly caused by the antibiotics pristinamycin and aminopenicillins, followed by quinolones, (hydroxy-)chloroquine, and sulfonamides. DRESS can be induced by a number of drugs known to cause SJS/TEN, such as certain antiepileptics and allopurinol, but also other medications (e.g., minocyclin).
Keywords: Stevens-Johnson syndrome; acute generalized exanthematous pustulosis; cutaneous adverse drug reactions; drug exanthema; drug reaction with eosinophilia and systemic symptoms; epidemiology; toxic epidermal necrolysis.
Figures

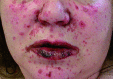
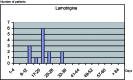

Republished from
- pp. 131-144 doi: 10.5414/ALX01508
References
-
- Auquier-Dunant A Mockenhaupt M Naldi L Correia O Schröder W Roujeau J-C Roujeau JC for the SCAR Study Group: Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Arch Dermatol. 2002; 138: 1019–1024. - PubMed
-
- Bastuji-Garin S Rzany B Stern RS Shear NH Naldi L Roujeau JC Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129: 92–96. - PubMed
-
- Bork K, Severe Cutaneous adverse drug reactions In:Burgdorf WHC, Plewig G, Wolff HH, Landthaler M, editors Braun-Falco’s Dermatology, 3. Heidelberg:Springer; 2009. p.456-472.
-
- Fagot JP Mockenhaupt M Bouwes Bavinck JN Naldi L Viboud C Roujeau JC Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis: preliminary results of a case-control study. AIDS. 2001; 15: 1–6. - PubMed
-
- Fiszenson-Albala F Auzerie V Mahe E Farinotti R Durand-Stocco C Crickx B Descamps V A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003; 149: 1018–1022. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
