M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study
- PMID: 30402880
- PMCID: PMC6587432
- DOI: 10.1002/cpt.1276
M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study
Abstract
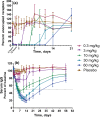
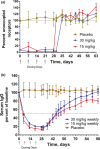
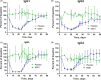
M281 is a fully human, anti-neonatal Fc receptor (FcRn) antibody that inhibits FcRn-mediated immunoglobulin G (IgG) recycling to decrease pathogenic IgG while preserving IgG production. A randomized, double-blind, placebo-controlled, first-in-human study with 50 normal healthy volunteers was designed to probe safety and the physiological maximum for reduction of IgG. Intravenous infusion of single ascending doses up to 60 mg/kg induced dose-dependent serum IgG reductions, which were similar across all IgG subclasses. Multiple weekly doses of 15 or 30 mg/kg achieved mean IgG reductions of ≈85% from baseline and maintained IgG reductions ≥75% from baseline for up to 24 days. M281 was well tolerated, with no serious or severe adverse events (AEs), few moderate AEs, and a low incidence of infection-related AEs similar to placebo treatment. The tolerability and consistency of M281 pharmacokinetics and pharmacodynamics support further evaluation of M281 in diseases mediated by pathogenic IgG.
© 2018 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
L.E.L., L.M., J.D., A.M.M., and S.A. are full‐time employees of Momenta Pharmaceuticals and may own stock or stock options. J.L.H. was an employee of Momenta Pharmaceuticals when the study was designed and conducted and subsequently a paid consultant of Momenta Pharmaceuticals and may own stock or stock options of Momenta Pharmaceuticals. N.A.C. was a full‐time employee of Momenta Pharmaceuticals during the execution of the study and subsequently a paid consultant of Momenta Pharmaceuticals, and owned stock, restricted stock, and stock options in Momenta Pharmaceuticals during manuscript preparation. R.G.T., T.B., and M.P.v.I. are full‐time employees of PRA Health Sciences and may own stock or stock options in PRA Health Sciences. D.J.N. was principal of Drug Development Consulting, currently is a full‐time employee of Certara Strategic Consulting and is a paid consultant of Momenta Pharmaceuticals. J.B.S. is principal at Streisand Biomedical Consulting and a paid consultant of Momenta Pharmaceuticals.
Figures
References
-
- Eggert, M. , Zettl, U.K. & Neeck, G. Autoantibodies in autoimmune diseases. Curr. Pharm. Des. 16, 1634–1643 (2010). - PubMed
-
- Giacomelli, R. et al International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)? The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun. Rev. 16, 911–924 (2017). - PubMed
-
- Winthrop, K.L. et al The unmet need in rheumatology: reports from the targeted therapies meeting 2017. Clin. Immunol. 186, 87–93 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

