Development of new 3D human ex vivo models to study sebaceous gland lipid metabolism and modulations
- PMID: 30402911
- PMCID: PMC6430446
- DOI: 10.1111/cpr.12524
Development of new 3D human ex vivo models to study sebaceous gland lipid metabolism and modulations
Abstract
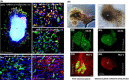
Objectives: Sebaceous glands maintain skin homeostasis by producing sebum. Low production can induce hair loss and fragile skin. Overproduction provokes seborrhoea and may lead to acne and inflammatory events. To better study sebaceous gland maintenance, sebocyte maturation, lipid production and ageing or inflammatory processes, we developed innovative 3D ex vivo models for human sebaceous glands.
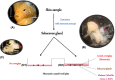
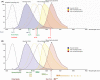
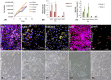
Materials and methods: Culture conditions and analytical methods optimized on sebocyte monolayers were validated on extracted sebaceous glands and allowed the development of two 3D models: (a) "air-liquid" interface and (b) human fibronectin-coated "sandwich" method. Lipid production was assessed with microscopy, fluorometry or flow cytometry analysis after Nile Red staining. Specific lipids (particularly squalene and peroxidized squalene) were measured by Gas or liquid Chromatography and Mass spectrometry.
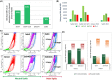
Results: This study allowed us to select appropriate conditions and design Seb4Gln culture medium inducing sebocyte proliferation and neutral lipid production. The "air-liquid" model was appropriate to induce sebocyte isolation. The "sandwich" model enabled sebaceous gland maintenance up to 42 days. A treatment with Insulin Growth Factor-1 allowed validation of the model as we succeeded in mimicking dynamic lipid overproduction.
Conclusion: Functional sebocyte maturation and physiological maintenance were preserved up to 6 weeks in our models. Associated with functional assays, they provide a powerful platform to mimic physiological skin lipid metabolism and to screen for active ingredients modulating sebum production.
Keywords: 3D model; sebaceous gland; sebocyte; sebum; skin lipid metabolism; squalene.
© 2018 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare they have no conflict of interest related to this study.
Figures

Similar articles
-
Melanocortin-5 receptor and sebogenesis.Eur J Pharmacol. 2011 Jun 11;660(1):202-6. doi: 10.1016/j.ejphar.2010.10.100. Epub 2011 Jan 6. Eur J Pharmacol. 2011. PMID: 21215742 Review.
-
Sebaceous gland: Milestones of 30-year modelling research dedicated to the "brain of the skin".Exp Dermatol. 2020 Nov;29(11):1069-1079. doi: 10.1111/exd.14184. Epub 2020 Sep 25. Exp Dermatol. 2020. PMID: 32875660
-
Acne and sebaceous gland function.Clin Dermatol. 2004 Sep-Oct;22(5):360-6. doi: 10.1016/j.clindermatol.2004.03.004. Clin Dermatol. 2004. PMID: 15556719 Review.
-
Sebum lipids influence macrophage polarization and activation.Br J Dermatol. 2017 Dec;177(6):1671-1682. doi: 10.1111/bjd.15754. Epub 2017 Nov 28. Br J Dermatol. 2017. PMID: 28646583
-
Human sebum requires de novo lipogenesis, which is increased in acne vulgaris and suppressed by acetyl-CoA carboxylase inhibition.Sci Transl Med. 2019 May 15;11(492):eaau8465. doi: 10.1126/scitranslmed.aau8465. Sci Transl Med. 2019. PMID: 31092695 Clinical Trial.
Cited by
-
Serum lipidomics-based study of electroacupuncture for skin wound repair in rats.J Cell Mol Med. 2023 Oct;27(20):3127-3146. doi: 10.1111/jcmm.17891. Epub 2023 Jul 30. J Cell Mol Med. 2023. PMID: 37517065 Free PMC article.
-
Aging in the sebaceous gland.Front Cell Dev Biol. 2022 Aug 17;10:909694. doi: 10.3389/fcell.2022.909694. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36060807 Free PMC article. Review.
-
The World's First Acne Dysbiosis-like Model of Human 3D Ex Vivo Sebaceous Gland Colonized with Cutibacterium acnes and Staphylococcus epidermidis.Microorganisms. 2023 Aug 29;11(9):2183. doi: 10.3390/microorganisms11092183. Microorganisms. 2023. PMID: 37764027 Free PMC article.
References
-
- Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271‐281. - PubMed
-
- Borchman D, Yappert MC, Milliner SE, Smith RJ, Bhola R. Confirmation of the presence of squalene in human eyelid lipid by heteronuclear single quantum correlation spectroscopy. Lipids. 2013;48:1269‐1277. - PubMed
-
- Schneider M, Zouboulis C. Primary sebocytes and sebaceous gland cell lines for studying sebaceous lipogenesis and sebaceous gland diseases. Exp Dermatol. 2018. - PubMed
-
- Dahloff M, Camera E, Schäffer M, et al. Schneider MR/ Sebaceous lipids are essential for water repulsion, protection against UVB‐induced apoptosis and ocular integrity in mice. Development. 2016;143:1823‐1831. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

