Distinct effects of (R)-modafinil and its (R)- and (S)-fluoro-analogs on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats
- PMID: 30402972
- PMCID: PMC8294075
- DOI: 10.1111/ejn.14256
Distinct effects of (R)-modafinil and its (R)- and (S)-fluoro-analogs on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats
Abstract
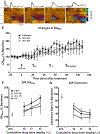
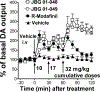
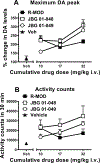
Psychostimulant use disorders remain an unabated public health concern worldwide, but no FDA approved medications are currently available for treatment. Modafinil (MOD), like cocaine, is a dopamine reuptake inhibitor and one of the few drugs evaluated in clinical trials that has shown promise for the treatment of cocaine or methamphetamine use disorders in some patient subpopulations. Recent structure-activity relationship and preclinical studies on a series of MOD analogs have provided insight into modifications of its chemical structure that may lead to advancements in clinical efficacy. Here, we have tested the effects of the clinically available (R)-enantiomer of MOD on extracellular dopamine levels in the nucleus accumbens shell, a mesolimbic dopaminergic projection field that plays significant roles in various aspects of psychostimulant use disorders, measured in vivo by fast-scan cyclic voltammetry and by microdialysis in Sprague-Dawley rats. We have compared these results with those obtained under identical experimental conditions with two novel and enantiopure bis(F) analogs of MOD, JBG1-048 and JBG1-049. The results show that (R)-modafinil (R-MOD), JBG1-048, and JBG1-049, when administered intravenously with cumulative drug-doses, will block the dopamine transporter and reduce the clearance rate of dopamine, increasing its extracellular levels. Differences among the compounds in their maximum stimulation of dopamine levels, and in their time course of effects were also observed. These data highlight the mechanistic underpinnings of R-MOD and its bis(F) analogs as pharmacological tools to guide the discovery of novel medications to treat psychostimulant use disorders.
Keywords: addiction; cocaine use disorder; dopamine microdialysis; fast-scan cyclic voltammetry; nucleus accumbens shell.
Published 2018. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Authors’ competing interest.
All Authors declare that there are not competing financial interests of any kind in relation to the work described in the present manuscript.
Figures
Similar articles
-
Effects of ( R)-Modafinil and Modafinil Analogues on Dopamine Dynamics Assessed by Voltammetry and Microdialysis in the Mouse Nucleus Accumbens Shell.ACS Chem Neurosci. 2019 Apr 17;10(4):2012-2021. doi: 10.1021/acschemneuro.8b00340. Epub 2019 Jan 31. ACS Chem Neurosci. 2019. PMID: 30645944 Free PMC article.
-
Dual DAT and sigma receptor inhibitors attenuate cocaine effects on nucleus accumbens dopamine dynamics in rats.Eur J Neurosci. 2024 May;59(10):2436-2449. doi: 10.1111/ejn.16293. Epub 2024 Mar 5. Eur J Neurosci. 2024. PMID: 38444104 Free PMC article.
-
R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse.Biol Psychiatry. 2012 Sep 1;72(5):405-13. doi: 10.1016/j.biopsych.2012.03.022. Epub 2012 Apr 25. Biol Psychiatry. 2012. PMID: 22537794 Free PMC article.
-
Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder.Curr Opin Pharmacol. 2021 Feb;56:13-21. doi: 10.1016/j.coph.2020.07.007. Epub 2020 Sep 11. Curr Opin Pharmacol. 2021. PMID: 32927246 Free PMC article. Review.
-
Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors.Molecules. 2023 Jul 7;28(13):5270. doi: 10.3390/molecules28135270. Molecules. 2023. PMID: 37446929 Free PMC article. Review.
Cited by
-
Microwave coprocessing of modafinil with Gelucire®: Thermal and compression characteristics.ADMET DMPK. 2025 Jan 19;13(1):2569. doi: 10.5599/admet.2569. eCollection 2025. ADMET DMPK. 2025. PMID: 40161887 Free PMC article.
-
Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap?Front Neurosci. 2021 May 26;15:656475. doi: 10.3389/fnins.2021.656475. eCollection 2021. Front Neurosci. 2021. PMID: 34121988 Free PMC article. Review.
-
Addressing the instability issue of dopamine during microdialysis: the determination of dopamine, serotonin, methamphetamine and its metabolites in rat brain.J Chromatogr A. 2020 Sep 13;1627:461403. doi: 10.1016/j.chroma.2020.461403. Epub 2020 Jul 13. J Chromatogr A. 2020. PMID: 32823108 Free PMC article.
-
Structure-activity relationships for a series of (Bis(4-fluorophenyl)methyl)sulfinylethyl-aminopiperidines and -piperidine amines at the dopamine transporter: Bioisosteric replacement of the piperazine improves metabolic stability.Eur J Med Chem. 2020 Dec 15;208:112674. doi: 10.1016/j.ejmech.2020.112674. Epub 2020 Aug 9. Eur J Med Chem. 2020. PMID: 32947229 Free PMC article.
-
Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders.Neuropsychopharmacology. 2019 Jul;44(8):1435-1444. doi: 10.1038/s41386-019-0366-z. Epub 2019 Mar 11. Neuropsychopharmacology. 2019. PMID: 30858517 Free PMC article.
References
-
- Adler CH, Caviness JN, Hentz JG, Lind M & Tiede J (2003) Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord, 18, 287–293. - PubMed
-
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E & Elkashef AM (2012) Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend, 120, 135–141. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z1A DA000389 21/National Institute on Drug Abuse - Intramural Research Program, NIH/DHHS/International
- ZIA DA000611/ImNIH/Intramural NIH HHS/United States
- Z1A DA000611 2/National Institute on Drug Abuse - Intramural Research Program, NIH/DHHS/International
- ZIA DA000389/ImNIH/Intramural NIH HHS/United States
- K02 DA000389/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

