Factors Affecting Hydrogen Atom Transfer Reactivity of Metal-Oxo Porphyrinoid Complexes
- PMID: 30403479
- PMCID: PMC6676912
- DOI: 10.1021/acs.accounts.8b00414
Factors Affecting Hydrogen Atom Transfer Reactivity of Metal-Oxo Porphyrinoid Complexes
Abstract
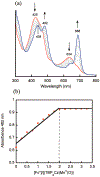
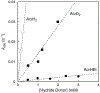
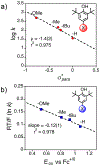
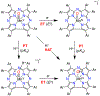
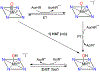
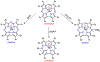
There has been considerable interest in hydrogen atom transfer (HAT) reactions mediated by metal/oxygen species because of their central role in metalloenzyme function as well as synthetic catalysts. This Account focuses on our progress in synthesizing high-valent metal-oxo and metal-hydroxo porphyrinoid complexes and determining their reactivities in a range of HAT processes. For these studies we have utilized corrolazine and corrole ligands, which are a ring-contracted subclass of porphyrinoid compounds designed to stabilize high-valent metal complexes. The high-valent manganese complex MnV(O)(TBP8Cz) (TBP8Cz = octakis(4- tert-butylphenyl)corrolazine3-) provided an early example of a well-characterized low-potential oxidant that can still be effective at abstracting H atoms from certain C-H/O-H bonds. Approximating the thermodynamics of the HAT reactivity of the MnV(O) complex and related species with the help of a square scheme approach, in which HAT can be formally separated into proton (p Ka) and electron transfers ( E°), indicates that affinity for the proton (i.e., the basicity) is a key factor in promoting HAT. Anionic axial ligands have a profound influence on the HAT reactivity of MnV(O)(TBP8Cz), supporting the conclusion that basicity is a critical parameter in determining the reactivity. The influence of Lewis acids on MnV(O)(TBP8Cz) was examined, and it was shown that both the electronic structure and reactivity toward HAT were significantly altered. High-valent Cr(O), Re(O), and Fe(O) corrolazines were prepared, and a range of HAT reactions were studied with these complexes. The chromium and manganese complexes form a rare pair of structurally characterized CrV(O) and MnV(O) species in identical ligand environments, allowing for a direct comparison of their HAT reactivities. Although the CrV(O) species was the better oxidant as measured by redox potentials, the MnV(O) species was significantly more reactive in HAT oxidations, pointing again to basicity as a key determinant of HAT reactivity. The iron complex, FeIV(O)(TBP8Cz+•), is an analogue of the heme enzyme Compound I intermediate, and was found to be mildly reactive toward H atom abstraction from C-H bonds. In contrast, ReV(O)(TBP8Cz) was inert toward HAT, although one-electron oxidation to ReV(O)(TBP8Cz+•) led to some interesting reactivity mediated by the π-radical-cation ligand alone. Other ligand modifications, including peripheral substitution as well as novel alkylation of the meso position on the Cz core, were examined for their influence on HAT. A highly sterically encumbered corrole, tris(2,4,6-triphenylphenyl)corrole (ttppc), was employed for the isolation and structural characterization of the first MnIV(OH) complex in a porphyrinoid environment, MnIV(OH)(ttppc). This complex was highly reactive in HAT with O-H substrates and was found to be much more reactive than its higher-oxidation-state counterpart MnV(O)(ttppc), providing important mechanistic insights. These studies provided fundamental knowledge on the relationship between structure and function in high-valent M(O) and M(OH) models of heme enzyme reactivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


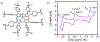
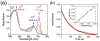

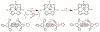

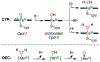
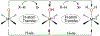



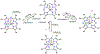

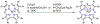
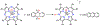
References
-
- Rittle J; Green MT Cytochrome P450 Compound I: Capture, Characterization, and C–H Bond Activation Kinetics. Science 2010, 330, 933–937. - PubMed
-
- Umena Y; Kawakami K; Shen JR; Kamiya N Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A. Nature 2011, 473, 55–60. - PubMed
-
- Cox N; Retegan M; Neese F; Pantazis DA; Boussac A; Lubitz W Electronic structure of the oxygen- evolving complex in photosystem II prior to O-O bond formation. Science 2014, 345, 804–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

