Primary structures of different isoforms of buffalo pregnancy-associated glycoproteins (BuPAGs) during early pregnancy and elucidation of the 3-dimensional structure of the most abundant isoform BuPAG 7
- PMID: 30403702
- PMCID: PMC6221303
- DOI: 10.1371/journal.pone.0206143
Primary structures of different isoforms of buffalo pregnancy-associated glycoproteins (BuPAGs) during early pregnancy and elucidation of the 3-dimensional structure of the most abundant isoform BuPAG 7
Abstract
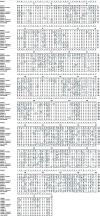
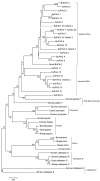
Pregnancy-associated glycoproteins (PAGs) are expressed during pregnancy by the trophoectodermal cells of fetus. Presence of PAGs in dam's circulation has been widely used in pregnancy diagnosis. The present study reports the identification and characterization of different PAG isoforms in buffalo during early stages of pregnancy. The PAG mRNAs isolated from fetal cotyledons (Pregnancy stages: 45, 75 and 90 days) were successfully cloned in pJET1.2 vector and transformed in E. coli. A total of 360 random clones were sequenced and correlated with their stages of expression. A total of 12 isoforms namely, BuPAG 1, 2, 4, 6, 7, 8, 9, 13, 15, 16, 18 and one new isoform were identified. BuPAG 7 was found as the most abundant isoform in all three stages followed by BuPAG 18. Further, a large number of variants were found for most of these isoforms. Phylogenetic relationship of identified BuPAGs showed that BuPAG 2 belonged to an ancient group while other members clustered with modern group. Three-dimensional (3D) structure of BuPAG 7 was determined by homology modeling and molecular dynamic (MD) simulations which displayed a typical fold represented by other aspartic proteinase (AP) family members. Molecular docking of Pepstatin inhibitor with BuPAG 7 revealed to interact through various hydrogen bonding and hydrophobic interactions. Various amino acid substitutions were observed in peptide-binding cleft of BuPAG 7. Superimposition of BuPAG 7 with homologous structures revealed the presence of a 35-41 amino acid long insertion (alpha helix connected by two loops) near the N- terminus which seems to be a unique feature of BuPAG 7 in AP family. This is the first report on identification and sequence characterization of PAG isoforms in buffalo with unique finding that these isoforms represent many transcript variants. We also report 3D structure of the most abundant isoform BuPAG 7 for the first time.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Zoli AP, Demez P, Beckers JF, Reznik M, Beckers A. Light and electron microscopic immunolocalization of bovine pregnancy-associated glycoprotein in the bovine placentome. Biol Reprod. 1992a;46: 623–629. - PubMed
-
- Zoli AP, Guilbault LA, Delabaut P, Orates WEB, Beckers JF. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: its application for pregnancy diagnosis. Biol Reprod. 1992b;46: 83–92. - PubMed
-
- Xie S, Low BG, Nagel RJ, Beckers JF, Roberts RM. A novel glycoprotein of the aspartic proteinase gene family expressed in bovine placental trophectoderm. Biol Reprod. 1994;51: 1145–1153. - PubMed
-
- Green JA, Xie S, Quan X, Bao B, Gan X, Mathialagan N, et al. Pregnancy associated glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol Reprod. 2000;62: 1624–1631. - PubMed
-
- Weems YS, Lammoglia MA, Vera-Avila HR, Randel RD, King C, Sasser RG, et al. Effect of luteinizing hormone (LH), PGE2, 8-EPI-PGE1, 8-EPI-PGE2, trichosanthin, and pregnancy specific protein B (PSPB) on secretion of progesterone in vitro by corpora lutea (CL) from nonpregnant and pregnant cows. Prostaglandins Other Lipid Mediat. 1998;55(1): 27–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

