Structure of the Golgi apparatus is not influenced by a GAG deletion mutation in the dystonia-associated gene Tor1a
- PMID: 30403723
- PMCID: PMC6221310
- DOI: 10.1371/journal.pone.0206123
Structure of the Golgi apparatus is not influenced by a GAG deletion mutation in the dystonia-associated gene Tor1a
Abstract
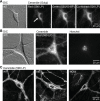
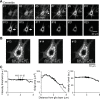
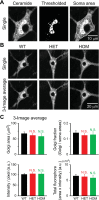
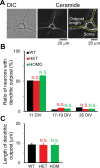
Autosomal-dominant, early-onset DYT1 dystonia is associated with an in-frame deletion of a glutamic acid codon (ΔE) in the TOR1A gene. The gene product, torsinA, is an evolutionarily conserved AAA+ ATPase. The fact that constitutive secretion from patient fibroblasts is suppressed indicates that the ΔE-torsinA protein influences the cellular secretory machinery. However, which component is affected remains unclear. Prompted by recent reports that abnormal protein trafficking through the Golgi apparatus, the major protein-sorting center of the secretory pathway, is sometimes associated with a morphological change in the Golgi, we evaluated the influence of ΔE-torsinA on this organelle. Specifically, we examined its structure by confocal microscopy, in cultures of striatal, cerebral cortical and hippocampal neurons obtained from wild-type, heterozygous and homozygous ΔE-torsinA knock-in mice. In live neurons, the Golgi was assessed following uptake of a fluorescent ceramide analog, and in fixed neurons it was analyzed by immuno-fluorescence staining for the Golgi-marker GM130. Neither staining method indicated genotype-specific differences in the size, staining intensity, shape or localization of the Golgi. Moreover, no genotype-specific difference was observed as the neurons matured in vitro. These results were supported by a lack of genotype-specific differences in GM130 expression levels, as assessed by Western blotting. The Golgi was also disrupted by treatment with brefeldin A, but no genotype-specific differences were found in the immuno-fluorescence staining intensity of GM130. Overall, our results demonstrate that the ΔE-torsinA protein does not drastically influence Golgi morphology in neurons, irrespective of genotype, brain region (among those tested), or maturation stage in culture. While it remains possible that functional changes in the Golgi exist, our findings imply that any such changes are not severe enough to influence its morphology to a degree detectable by light microscopy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures











Similar articles
-
Dystonia-associated protein torsinA is not detectable at the nerve terminals of central neurons.Neuroscience. 2013 Dec 3;253:316-29. doi: 10.1016/j.neuroscience.2013.08.060. Epub 2013 Sep 8. Neuroscience. 2013. PMID: 24025868
-
Miniature release events of glutamate from hippocampal neurons are influenced by the dystonia-associated protein torsinA.Synapse. 2012 Sep;66(9):807-22. doi: 10.1002/syn.21571. Epub 2012 Jun 20. Synapse. 2012. PMID: 22588999
-
Synaptic vesicle recycling is enhanced by torsinA that harbors the DYT1 dystonia mutation.Synapse. 2012 May;66(5):453-64. doi: 10.1002/syn.21534. Epub 2012 Feb 15. Synapse. 2012. PMID: 22213465
-
The role of torsinA in dystonia.Eur J Neurol. 2010 Jul;17 Suppl 1:81-7. doi: 10.1111/j.1468-1331.2010.03057.x. Eur J Neurol. 2010. PMID: 20590813 Review.
-
TorsinA and dystonia: from nuclear envelope to synapse.J Neurochem. 2009 Jun;109(6):1596-609. doi: 10.1111/j.1471-4159.2009.06095.x. Epub 2009 Apr 8. J Neurochem. 2009. PMID: 19457118 Review.
Cited by
-
Unraveling the Multifaceted Role of the Golgi Apparatus: Insights into Neuronal Plasticity, Development, Neurogenesis, Alzheimer's Disease, and SARS-CoV-2 Interactions.Brain Sci. 2023 Sep 23;13(10):1363. doi: 10.3390/brainsci13101363. Brain Sci. 2023. PMID: 37891732 Free PMC article. Review.
-
The Role of GM130 in Nervous System Diseases.Front Neurol. 2021 Oct 28;12:743787. doi: 10.3389/fneur.2021.743787. eCollection 2021. Front Neurol. 2021. PMID: 34777211 Free PMC article. Review.
-
Herpes Simplex Virus Organizes Cytoplasmic Membranes To Form a Viral Assembly Center in Neuronal Cells.J Virol. 2020 Sep 15;94(19):e00900-20. doi: 10.1128/JVI.00900-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32699089 Free PMC article.
-
Acat1/Soat1 knockout extends the mutant Npc1 mouse lifespan and ameliorates functional deficiencies in multiple organelles of mutant cells.Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2201646119. doi: 10.1073/pnas.2201646119. Epub 2022 May 4. Proc Natl Acad Sci U S A. 2022. PMID: 35507892 Free PMC article.
-
Cooperative lipolytic control of neuronal triacylglycerol by spastic paraplegia-associated enzyme DDHD2 and ATGL.J Lipid Res. 2023 Nov;64(11):100457. doi: 10.1016/j.jlr.2023.100457. Epub 2023 Oct 11. J Lipid Res. 2023. PMID: 37832604 Free PMC article.
References
-
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40(2):277–95. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

