In vitro axenic germination and cultivation of mixotrophic Pyroloideae (Ericaceae) and their post-germination ontogenetic development
- PMID: 30403767
- PMCID: PMC6417480
- DOI: 10.1093/aob/mcy195
In vitro axenic germination and cultivation of mixotrophic Pyroloideae (Ericaceae) and their post-germination ontogenetic development
Abstract
Background and aims: Pyroloids, forest sub-shrubs of the Ericaceae family, are an important model for their mixotrophic nutrition, which mixes carbon from photosynthesis and from their mycorrhizal fungi. They have medical uses but are difficult to cultivate ex situ; in particular, their dust seeds contain undifferentiated, few-celled embryos, whose germination is normally fully supported by fungal partners. Their germination and early ontogenesis thus remain elusive.
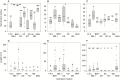
Methods: An optimized in vitro cultivation system of five representatives from the subfamily Pyroloideae was developed to study the strength of seed dormancy and the effect of different media and conditions (including light, gibberellins and soluble saccharides) on germination. The obtained plants were analysed for morphological, anatomical and histochemical development.
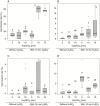
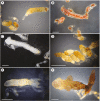
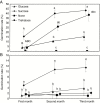
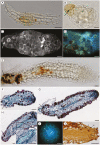
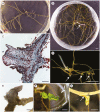
Key results: Thanks to this novel cultivation method, which breaks dormancy and achieved up to 100 % germination, leafy shoots were obtained in vitro for representatives of all pyroloid genera (Moneses, Orthilia, Pyrola and Chimaphila). In all cases, the first post-germination stage is an undifferentiated structure, from which a root meristem later emerges, well before formation of an adventive shoot.
Conclusions: This cultivation method can be used for further research or for ex situ conservation of pyroloid species. After strong seed dormancy is broken, the tiny globular embryo of pyroloids germinates into an intermediary zone, which is functionally convergent with the protocorm of other plants with dust seeds such as orchids. Like the orchid protocorm, this intermediary zone produces a single meristem: however, unlike orchids, which produce a shoot meristem, pyroloids first generate a root meristem.
Keywords: Chimaphila; Moneses; Monotropa; Pyrola; in vitro culture; Ericaceae; convergent evolution; mixotrophy; orchid; protocorm; seed dormancy; seed germination.
© The Author(s) 2018. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Arditti J. 1967. Factors affecting the germination of orchid seeds. Botanical Review 33: 1–97.
-
- Arditti J. 2008. Micropropagation of orchids, Vol. 1, 2nd edn. Chichester, UK: John Wiley and Sons.
-
- Arditti J, Ghani A. 2000. Numerical and physical properties of orchid seeds and their biological implications. New Phytologist 145: 367–421. - PubMed
-
- Bartlett M. 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Society A: Mathematical and Physical Sciences 160: 268–282.
-
- Bernard N. 1909. L’évolution dans la symbiose. Les Orchideés et leurs champignons commensaux. Annales des Sciences Naturelles Serie (Botanique) 9: 1–196.

