Fat-1 Transgene Is Associated With Improved Reproductive Outcomes
- PMID: 30403782
- PMCID: PMC6260063
- DOI: 10.1210/en.2018-00723
Fat-1 Transgene Is Associated With Improved Reproductive Outcomes
Abstract
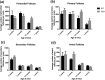
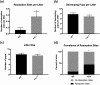
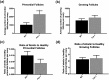
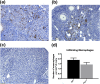
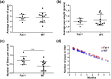
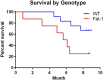
High intake of ω-3 polyunsaturated fatty acids (PUFAs) has been associated with a variety of health benefits. However, the role of ω-3 PUFAs in female reproductive function is unclear, with studies showing both positive and negative effects. The type of diet that ω-3 fatty acids are consumed with, for example, a balanced diet vs a high-fat diet (HFD), may influence how ω-3 fatty acids affect female reproductive function. To address the role of ω-3 PUFAs in female reproduction, we used the fat-1 mouse both with and without HFD exposure. Fat-1 mice constitutively express the fat-1 transgene, allowing the conversion of ω-6 to ω-3 fatty acids to yield an optimal tissue ratio of ω-6 to ω-3 fatty acids (∼1:1). In our study, at 15 weeks of age, fat-1 mice had elevated primordial follicles compared with wild-type controls with both standard chow and HFD feeding. Higher serum levels of the ω-3 docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and eicosapentaenoic acid (EPA) were positively associated with primordial follicle numbers, whereas the ratio of the ω-6 arachidonic acid to EPA + DPA + DHA had the opposite effect. Furthermore, fat-1 mice had increased pregnancy rates and shorter time to pregnancy when fed an HFD compared with wild-type mice. In conclusion, our novel preclinical model suggests that high tissue levels of long-chain ω-3 PUFAs are associated with an improved ovarian reserve and improved reproductive outcomes. Further studies are needed to evaluate ω-3 PUFAs as a potential intervention strategy in women with diminished ovarian reserve.
Figures

Similar articles
-
The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues.Int J Mol Sci. 2019 Nov 24;20(23):5895. doi: 10.3390/ijms20235895. Int J Mol Sci. 2019. PMID: 31771283 Free PMC article.
-
Increased ω-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with autism spectrum disorders.Life Sci. 2016 Jan 15;145:205-12. doi: 10.1016/j.lfs.2015.12.039. Epub 2015 Dec 24. Life Sci. 2016. PMID: 26724495
-
Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and women: The Kuopio Ischaemic Heart Disease Risk Factor Study.Eur J Clin Nutr. 2016 Aug;70(8):970-5. doi: 10.1038/ejcn.2016.59. Epub 2016 Apr 13. Eur J Clin Nutr. 2016. PMID: 27071510
-
A meta-analytic review of polyunsaturated fatty acid compositions in dementia.J Clin Psychiatry. 2012 Sep;73(9):1245-54. doi: 10.4088/JCP.11r07546. Epub 2012 Aug 7. J Clin Psychiatry. 2012. PMID: 22938939 Review.
-
Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity.Mediators Inflamm. 2021 Jan 2;2021:8879227. doi: 10.1155/2021/8879227. eCollection 2021. Mediators Inflamm. 2021. PMID: 33488295 Free PMC article. Review.
Cited by
-
Association between polyunsaturated fatty acid intake and estradiol levels among U.S. women.Front Nutr. 2024 Nov 20;11:1500705. doi: 10.3389/fnut.2024.1500705. eCollection 2024. Front Nutr. 2024. PMID: 39634551 Free PMC article.
-
High-fat diet-induced dysregulation of ovarian gene expression is restored with chronic omega-3 fatty acid supplementation.Mol Cell Endocrinol. 2020 Jan 1;499:110615. doi: 10.1016/j.mce.2019.110615. Epub 2019 Oct 16. Mol Cell Endocrinol. 2020. PMID: 31628964 Free PMC article.
-
Decreased fatty acids induced granulosa cell apoptosis in patients with diminished ovarian reserve.J Assist Reprod Genet. 2022 May;39(5):1105-1114. doi: 10.1007/s10815-022-02462-8. Epub 2022 Mar 26. J Assist Reprod Genet. 2022. PMID: 35347502 Free PMC article.
-
Detoxification Cytochrome P450s (CYPs) in Families 1-3 Produce Functional Oxylipins from Polyunsaturated Fatty Acids.Cells. 2022 Dec 24;12(1):82. doi: 10.3390/cells12010082. Cells. 2022. PMID: 36611876 Free PMC article. Review.
-
Genome-wide association and genomic prediction for a reproductive index summarizing fertility outcomes in U.S. Holsteins.G3 (Bethesda). 2023 Aug 30;13(9):jkad043. doi: 10.1093/g3journal/jkad043. G3 (Bethesda). 2023. PMID: 36848195 Free PMC article.
References
-
- Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112(4):298–304. - PubMed
-
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201. - PubMed
-
- Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234(1–2):75–79. - PubMed
-
- Abayasekara DR, Wathes DC. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot Essent Fatty Acids. 1999;61(5):275–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

