MicroR408 regulates defense response upon wounding in sweet potato
- PMID: 30403812
- PMCID: PMC6322576
- DOI: 10.1093/jxb/ery381
MicroR408 regulates defense response upon wounding in sweet potato
Abstract
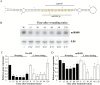
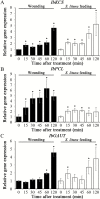
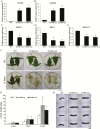
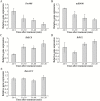
MiRNAs play diverse roles in plant development and defense responses by binding to their mRNA targets based on sequence complementarity. Here, we investigated a wound-related miR408 and its target genes in sweet potato (Ipomoea batatas) by small RNA deep sequencing and transcriptome analysis. The expression patterns of miR408 and the miR408 precursor were significantly repressed by wounding and jasmonate (JA). In contrast, expression of the putative target genes IbKCS (3-ketoacyl-CoA synthase 4), IbPCL (plantacyanin), and IbGAUT (galacturonosyltransferase 7-like) of miR408 was increased following wounding, whereas only IbKCS was increased after JA treatment. Target cleavage site mapping and Agrobacterium-mediated transient assay demonstrated that IbKCS, IbPCL, and IbGAUT were the targets of miR408. The expression of miR408 target genes was repressed in transgenic sweet potatoes overexpressing miR408. These data indicated a relationship between miR408 and its target genes. Notably, miR408-overexpressing plants showed a semi-dwarf phenotype and attenuated resistance to insect feeding, while transgenic plants overexpressing IbKCS exhibited more insect resistance than plants overexpressing only the empty vector. Collectively, sweet potato reduces the abundance of miR408 upon wounding to elevate the expression of IbKCS, IbPCL, and IbGAUT. The expression of IbKCS enhances the defense system against herbivore wounding.
Figures


References
-
- Ameres SL, Zamore PD. 2013. Diversifying microRNA sequence and function. Nature Reviews. Molecular Cell Biology 14, 475–488. - PubMed
-
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. - PubMed
-
- Axtell MJ, Bowman JL. 2008. Evolution of plant microRNAs and their targets. Trends in Plant Science 13, 343–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

