Design, Synthesis, and Biological Evaluation of Polyaminocarboxylate Ligand-Based Theranostic Conjugates for Antibody-Targeted Cancer Therapy and Near-Infrared Optical Imaging
- PMID: 30403833
- PMCID: PMC6324731
- DOI: 10.1002/cmdc.201800598
Design, Synthesis, and Biological Evaluation of Polyaminocarboxylate Ligand-Based Theranostic Conjugates for Antibody-Targeted Cancer Therapy and Near-Infrared Optical Imaging
Abstract
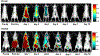
We report the design, synthesis, and evaluation of polyaminocarboxylate ligand-based antibody conjugates for potential application in targeted cancer therapy and near-infrared (NIR) fluorescence imaging. We synthesized a new polyaminocarboxylate chelate (CAB-NE3TA) as a potential anticancer agent. CAB-NE3TA displayed potent inhibitory activities against various cancer cell lines. We then designed a multifunctional theranostic platform (CAB-NE3TA-PAN-IR800) constructed on an epidermal growth factor receptor (EGFR)-targeted antibody (panitumumab, PAN) labeled with a NIR fluorescent dye. We also built the first atomistic model of the EGFR-PAN complex and loaded it with the cytotoxic CAB-NE3TA and the NIR dye. The therapeutic (CAB-NE3TA-PAN) and theranostic (CAB-NE3TA-PAN-IR800) conjugates were evaluated using an EGFR-positive A431 (human skin cancer) cell xenograft mouse model. Biodistribution studies using NIR fluorescence imaging demonstrated that the CAB-NE3TA-PAN labeled with the IR800 dye selectively targeted the A431 tumors in mice and resulted in prolonged retention in the tumor tissue and displayed excellent clearance in blood and normal organs. The therapeutic conjugate was capable of significantly inhibiting tumor growth, leading to nearly complete disappearance of tumors in the mice. The results of our pilot in vivo studies support further evaluation of the novel ligand-based therapeutic and theranostic conjugates for targeted iron chelation cancer therapy and imaging applications.
Keywords: antibody-drug conjugates; anticancer agents; fluorescence imaging; theranostic agents.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
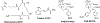








Similar articles
-
Theranostic Polyaminocarboxylate-Cyanine-Transferrin Conjugate for Anticancer Therapy and Near-Infrared Optical Imaging.ChemMedChem. 2016 Oct 6;11(19):2188-2193. doi: 10.1002/cmdc.201600072. Epub 2016 Sep 14. ChemMedChem. 2016. PMID: 27624789 Free PMC article.
-
Synthesis and evaluation of novel polyaminocarboxylate-based antitumor agents.J Med Chem. 2008 Apr 10;51(7):2208-15. doi: 10.1021/jm701307j. Epub 2008 Mar 18. J Med Chem. 2008. PMID: 18345610
-
Impact of C4'-O-Alkyl Linker on in Vivo Pharmacokinetics of Near-Infrared Cyanine/Monoclonal Antibody Conjugates.Mol Pharm. 2015 Sep 8;12(9):3303-11. doi: 10.1021/acs.molpharmaceut.5b00472. Epub 2015 Aug 18. Mol Pharm. 2015. PMID: 26261913 Free PMC article.
-
Future potential of osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes.Dalton Trans. 2018 Oct 30;47(42):14841-14854. doi: 10.1039/c8dt03432j. Dalton Trans. 2018. PMID: 30325378 Review.
-
A critical analysis of the potential of iron heterobimetallic complexes in anticancer research.J Inorg Biochem. 2025 Mar;264:112813. doi: 10.1016/j.jinorgbio.2024.112813. Epub 2024 Dec 15. J Inorg Biochem. 2025. PMID: 39794011 Review.
Cited by
-
Ligand-based active targeting strategies for cancer theranostics.Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3417-3441. doi: 10.1007/s00210-023-02612-4. Epub 2023 Jul 19. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37466702 Review.
-
Nanocarrier of α-Tocopheryl Succinate Based on a Copolymer Derivative of (4,7-dichloroquinolin-2-yl)methanol and Its Cytotoxicity against a Breast Cancer Cell Line.Polymers (Basel). 2023 Nov 7;15(22):4342. doi: 10.3390/polym15224342. Polymers (Basel). 2023. PMID: 38006067 Free PMC article.
-
Drug Combination in Cancer Treatment-From Cocktails to Conjugated Combinations.Cancers (Basel). 2021 Feb 7;13(4):669. doi: 10.3390/cancers13040669. Cancers (Basel). 2021. PMID: 33562300 Free PMC article. Review.
-
Polyamine-Drug Conjugates: Do They Boost Drug Activity?Molecules. 2023 Jun 2;28(11):4518. doi: 10.3390/molecules28114518. Molecules. 2023. PMID: 37298993 Free PMC article. Review.
References
-
- Lieu PT, Heiskala M, Peterson PA, Yang Y, Mol. Aspects Med. 2001, 22, 1–87. - PubMed
-
- Papanikolaou G, Pantopoulos K, Toxicol. Appl. Pharmacol. 2005, 202, 199–211. - PubMed
-
- Kalinowski DS, Richardson DR, Pharmacol. Rev. 2005, 57, 547–583. - PubMed
-
- Buss JL, Torti FM, Torti SV, Curr. Med. Chem. 2003, 10, 1021–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

