Human monocyte-derived macrophages inhibit HCMV spread independent of classical antiviral cytokines
- PMID: 30403913
- PMCID: PMC7000197
- DOI: 10.1080/21505594.2018.1535785
Human monocyte-derived macrophages inhibit HCMV spread independent of classical antiviral cytokines
Abstract
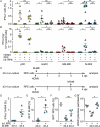
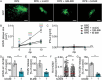
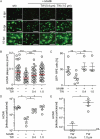
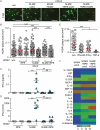
Infection of healthy individuals with human cytomegalovirus (HCMV) is usually unnoticed and results in life-long latency, whereas HCMV reactivation as well as infection of newborns or immunocompromised patients can cause life-threatening disease. To better understand HCMV pathogenesis we studied mechanisms that restrict HCMV spread. We discovered that HCMV-infected cells can directly trigger plasmacytoid dendritic cells (pDC) to mount antiviral type I interferon (IFN-I) responses, even in the absence of cell-free virus. In contrast, monocyte-derived cells only expressed IFN-I when stimulated by cell-free HCMV, or upon encounter of HCMV-infected cells that already produced cell-free virus. Nevertheless, also in the absence of cell-free virus, i.e., upon co-culture of infected epithelial/endothelial cells and monocyte-derived macrophages (moMΦ) or dendritic cells (moDC), antiviral responses were induced that limited HCMV spread. The induction of this antiviral effect was dependent on cell-cell contact, whereas cell-free supernatants from co-culture experiments also inhibited virus spread, implying that soluble factors were critically needed. Interestingly, the antiviral effect was independent of IFN-γ, TNF-α, and IFN-I as indicated by cytokine inhibition experiments using neutralizing antibodies or the vaccinia virus-derived soluble IFN-I binding protein B18R, which traps human IFN-α and IFN-β. In conclusion, our results indicate that human macrophages and dendritic cells can limit HCMV spread by IFN-I dependent as well as independent mechanisms, whereas the latter ones might be particularly relevant for the restriction of HCMV transmission via cell-to-cell spread.
Keywords: Human cytomegalovirus; epithelial cells; macrophages; plasmacytoid dendritic cells; type I interferons.
Figures

Similar articles
-
Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines.J Clin Invest. 1997 Dec 15;100(12):3154-63. doi: 10.1172/JCI119871. J Clin Invest. 1997. PMID: 9399963 Free PMC article.
-
cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells.PLoS Pathog. 2016 Apr 8;12(4):e1005546. doi: 10.1371/journal.ppat.1005546. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27058035 Free PMC article.
-
Growth of human cytomegalovirus in primary macrophages.Methods. 1998 Sep;16(1):126-38. doi: 10.1006/meth.1998.0650. Methods. 1998. PMID: 9774522
-
Cytomegalovirus persistence in macrophages and endothelial cells.Scand J Infect Dis Suppl. 1995;99:34-40. Scand J Infect Dis Suppl. 1995. PMID: 8668941 Review.
-
Control of Cytokines in Latent Cytomegalovirus Infection.Pathogens. 2020 Oct 21;9(10):858. doi: 10.3390/pathogens9100858. Pathogens. 2020. PMID: 33096622 Free PMC article. Review.
Cited by
-
Human cytomegalovirus exploits STING signaling and counteracts IFN/ISG induction to facilitate infection of dendritic cells.Nat Commun. 2024 Feb 26;15(1):1745. doi: 10.1038/s41467-024-45614-3. Nat Commun. 2024. PMID: 38409141 Free PMC article.
-
microRNA, a Subtle Indicator of Human Cytomegalovirus against Host Immune Cells.Vaccines (Basel). 2022 Jan 19;10(2):144. doi: 10.3390/vaccines10020144. Vaccines (Basel). 2022. PMID: 35214602 Free PMC article. Review.
-
Tuning the Orchestra: HCMV vs. Innate Immunity.Front Microbiol. 2020 Apr 15;11:661. doi: 10.3389/fmicb.2020.00661. eCollection 2020. Front Microbiol. 2020. PMID: 32351486 Free PMC article. Review.
-
Assessing Anti-HCMV Cell Mediated Immune Responses in Transplant Recipients and Healthy Controls Using a Novel Functional Assay.Front Cell Infect Microbiol. 2020 Jun 26;10:275. doi: 10.3389/fcimb.2020.00275. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670891 Free PMC article.
-
Human plasmacytoid dendritic cells mount a distinct antiviral response to virus-infected cells.Sci Immunol. 2021 Apr 2;6(58):eabc7302. doi: 10.1126/sciimmunol.abc7302. Sci Immunol. 2021. PMID: 33811059 Free PMC article.
References
-
- Griffiths P, Baraniak I, Reeves M.. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235:288–297. - PubMed
-
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–1779. - PubMed
-
- Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2014;54:1–5. - PubMed
